|
Research Article
Implementation of new protocol for pain management following cardiac surgery
1 Department of Anaesthesia, King’s College Hospital, London, United Kingdom, United Kingdom
2 Hull York Medical School, Hull, HU6 7RX, United Kingdom
3 Division of Plastic Surgery, Stanford University School of Medicine, Stanford, California 94305, United States
4 Department of Anaesthesia, King’s College Hospital, London, United Kingdom
5 Department of Anaesthesia, King’s College Hospital, London, United Kingdom
Address correspondence to:
Amy Rene Gomes
Hull York Medical School, Allam Medical Building, University of Hull, Hull, HU6 7RX,
United Kingdom
Message to Corresponding Author
Article ID: 100020A05ZM2020
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Milan Z, Gomes AR, Borrelli MR, Kunst G, Katyayni K. Implementation of new protocol for pain management following cardiac surgery. Edorium J Anesth 2020;6:100020A05ZM2020.ABSTRACT
Aims: We assessed the implementation and effectiveness of an updated protocol designed to improve pain management in cardiac surgery patients. The new updated protocol was recommended systemic pain assessment every four hours unless patients were unstable, using the numerical rating score (NRS) after the endotracheal extubation. Our secondary aim was to analyze the factors predicting patients’ postoperative pain to guide development of future pain management protocols.
Methods: Fifty patients undergoing cardiac surgery with median sternotomy were evaluated in this audit. Perioperative details and details regarding analgesic administration were collected. High-risk patients were classified as ones with a history of substance misuse, chronic pain, and preoperative opioid use. Pain was measured at rest, on coughing and on moving, for the first three postoperative days (POD), using 11-point NRS (0–10). Pain was considered “unacceptable” if it was NRS ≥4 at rest, and NRS ≥8 on activity. A univariate and multivariate mixed model linear regression was used to investigate factors that may contribute to pain following cardiac surgery.
Results: On POD1 38% of patients reported unacceptable pain at rest, and 27% reported unacceptable pain on coughing or moving. There was limited implementation of the new protocol, thus we cannot comment on the effectiveness of the updated protocol. Multivariate analysis demonstrated an overall significant interaction effect between postoperative day and risk (p = 0.032). It was found that high-risk patients reported pain to be greater than pain reported by low-risk patients on POD3 (2.14, CI −0.32 to 4.26, p = 0.054). Use of preoperative gabapentin did not affect pain at rest nor pain on coughing or moving (p > 0.5).
Conclusion: The new pain protocol was not followed in the majority of patient cases. Preoperatively, only 25 (56%) patients received gabapentin. No patients received patient-controlled analgesia (PCA) postoperatively. Seven (15%) patients identified as high risk received no differential pharmacological management contrary to the updated protocol. It is believed that e-mail is not sufficient to implement a new protocol such as this, thus resulting in protocol implementation failure. However, it was found that postoperative pain differed between high-and low-risk patients, especially at rest. This indicates that risk assessment and individualized pain protocols are important to optimize postoperative pain management following cardiac surgery. We have discussed the efforts required to improve future protocol implementation and pain management across disciplines.
Keywords: Audit, Cardiac, Pain, Postoperative, Protocol, Surgery
INTRODUCTION
Pain is common after cardiac surgery. A high proportion of cardiac surgery patients (30–75%) report moderate-to-severe acute postoperative pain [1] in the first days following surgery [2],[3]. Pain following cardiac surgery is multifactorial with different factors contributing to the pain experience at different times. While nociceptive pain from direct tissue trauma dominates the immediate postoperative period, musculoskeletal pain becomes unmasked around POD3 [4]. Thus, effective postoperative pain control is an essential component of care for the cardiac surgical patient, and is believed to facilitate recovery, improve morbidity, minimize costs of care, and increase overall patient satisfaction [4],[5].
A recent audit carried out on pain management in post-cardiac surgery patients in our institute [6] highlighted that 39% of our patients experienced unacceptable levels of pain: NRS ≥ 4 at rest and NRS ≥ 8 on POD1 and POD2. The high pain scores reported by patients in this audit prompted the revision of our post-cardiac surgery pain management protocol.
The primary aim of this audit was to (1) assess the implementation of the new pain management protocol, (2) its effectiveness in managing acute postoperative pain in cardiac surgery patients, and (3) explore factors predicting pain in this cohort in order to develop future postoperative pain management strategies.
METHODS
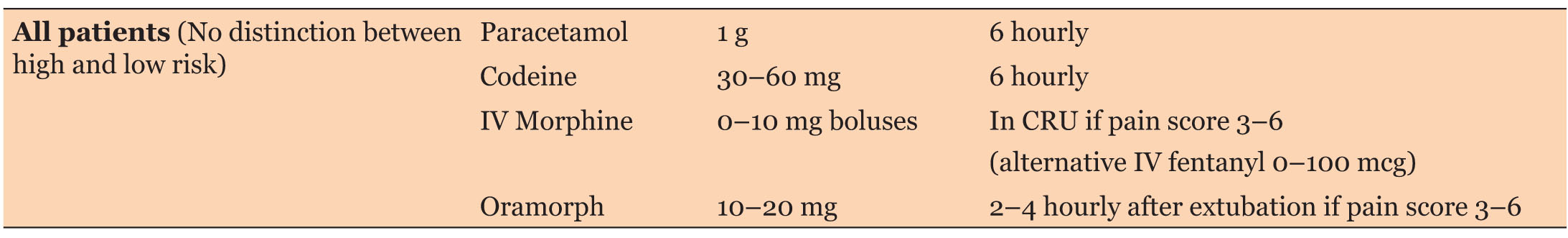
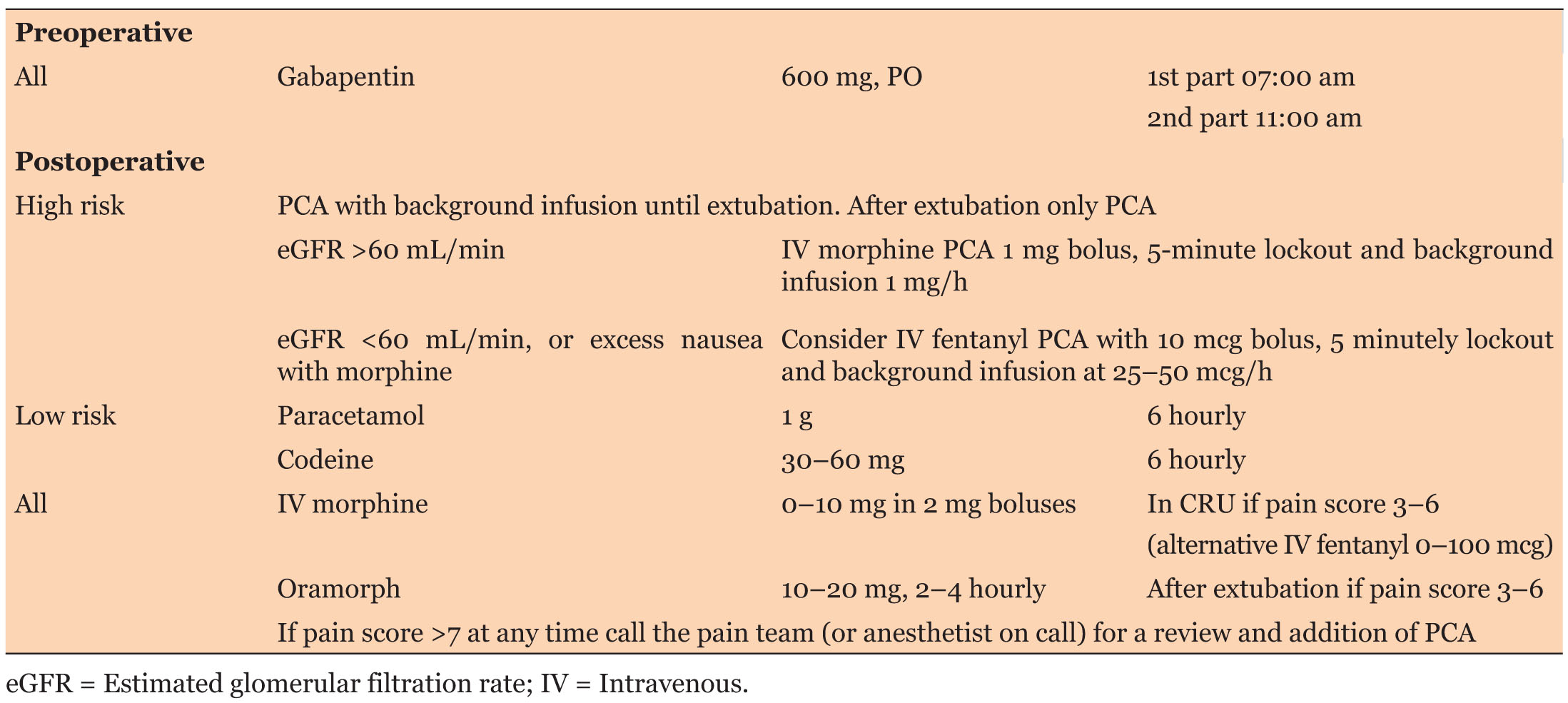
The original pain management protocol (Table 1) for patients undergoing cardiac surgery by sternotomy was updated by a team of cardiac anesthetist (Table 2). The protocol recommended systemic pain assessment every four hours unless unstable, using the NRS after tracheal extubation. The protocol was circulated among the cardiac surgery anesthetists via e-mail.
Patients with history of substance misuse, chronic pain, and preoperative opioid use were classified as high risk [7],[8],[9],[10],[11] for experiencing higher postoperative pain while all other patients were classified as low risk. Reported NRS scores ≥4 at rest and ≥8 on cough or movement were considered unacceptable.
Fifty consecutive patients who underwent elective and urgent cardiac surgery by a sternotomy were recruited for this study. The procedures were undertaken at our tertiary referral center between August and September 2017. Data regarding pain scores and pain management was collected by reviewing records and visiting patients every four hours following surgery. Data regarding demographic and operative details was collected prospectively from paper and electronic patient records maintained in our institute.
Data collected included patients’ Log Euro score, operation type, cardiopulmonary bypass and aortic cross clamp times, pre-and postoperative pharmacological analgesic treatment, and length of stay in intensive care unit (ITU) and hospital.
To collect the data regarding patients’ experience of postoperative pain, patients were visited by members of research team on the first three postoperative days and their pain levels were measured using the 11-point NRS. This scale ranges from 0 to 10, where 0 represents “no pain” and 10 represents “worst pain imaginable.” Patients were asked to rate their pain at rest, during coughing, and on movement every four hours.
Statistics
No formal sample size calculation was performed. It was hypothesized 50 patients would be adequate to obtain a good estimate of an average cardiac surgical patient’s pain given severe postoperative pain estimated to occur in 30–70%.
Descriptive statistics are reported as number of patients (percentages), means (with SDs), and medians (with ranges) where appropriate.
First, the intensity and incidence of postoperative pain was examined and a composite mean “activity pain score” was created for analysis. To identify the factors predictive of pain in the cardiac surgery patients, a generalized linear mixed-effects regression model was used. Factors were entered into a univariate analysis and those with a significance p < 0.3 were entered into the multivariate analysis. The factors included gender, age, pain scores on POD1, POD2, and POD3, predicted preoperative risk (high and low) and preoperative administration of gabapentin as a new feature of the new postoperative pain protocol. P < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS version 17.0 software (SPSS Inc, Chicago, IL, USA).
RESULTS
Demographic and surgical data
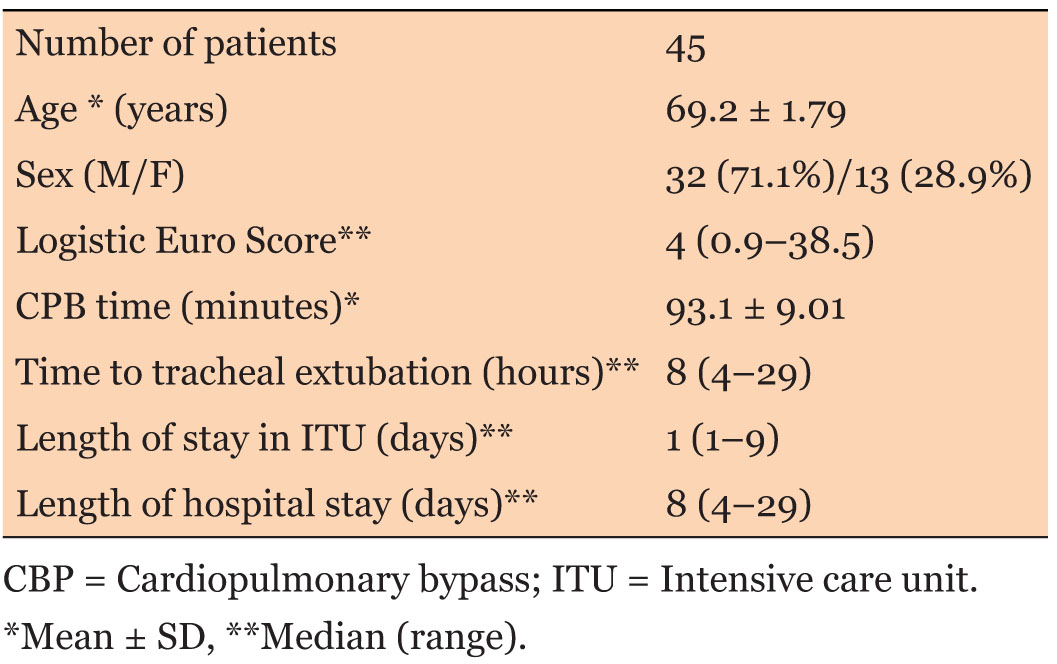
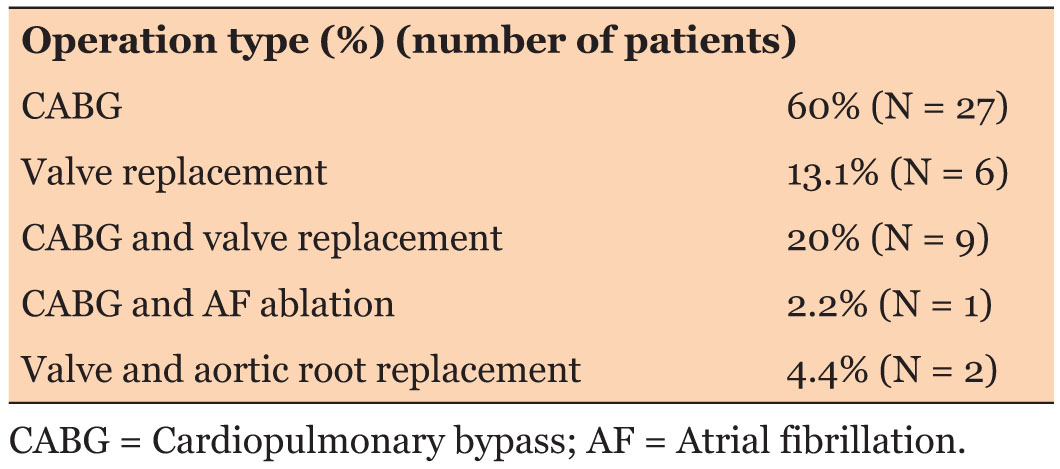
Fifty patients were initially included in the audit. Five patients had to be excluded from the study due to incomplete data (two patients) and prolonged intubation period more than three days (three patients). Thus, the final sample included 45 patients. Demographic and operative details are summarized in Table 3. The type of surgeries included is shown in Table 4.
Implementation of updated pain management protocol
Only 55.6% (N = 25) of patients received preoperative gabapentin. One sixth, or 15.7% (N = 7) of the patients were identified preoperatively “high-risk” for pain. There was no differential postoperative pharmacological management of high- and low-risk patients. No patients received patient-controlled analgesia (PCA) and despite NRS ≥ 8 on 36 occasions (mostly during coughing or moving), the pain team was not called.
Pain scores
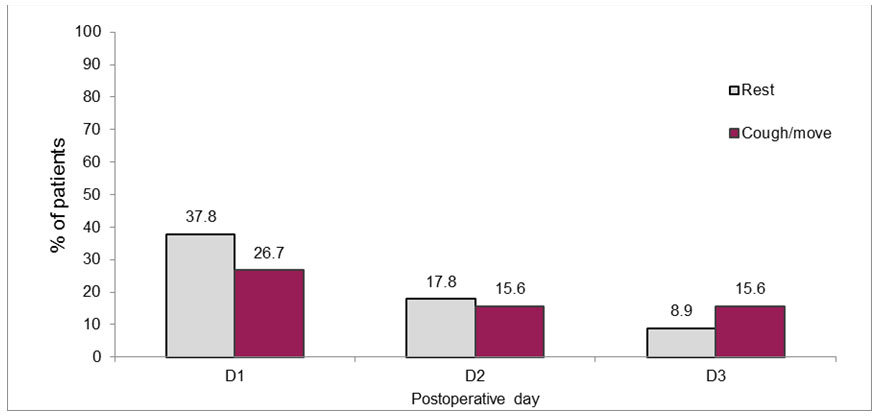
On POD1, 37.8% of patients reported “unacceptable” levels of rest pain (NRS ≥ 4), and 26.7% reported “unacceptable” pain on coughing or moving (NRS ≥ 8). The pain scores dropped on days 2 and 3 and 17.8% and 8.9% reported NRS ≥ 4, respectively. The percentage of patients reporting NRS ≥ 8 on coughing or moving also decreased with time to 15.6% on both POD2 and POD3 (Figure 1).
Comparison with previous audit
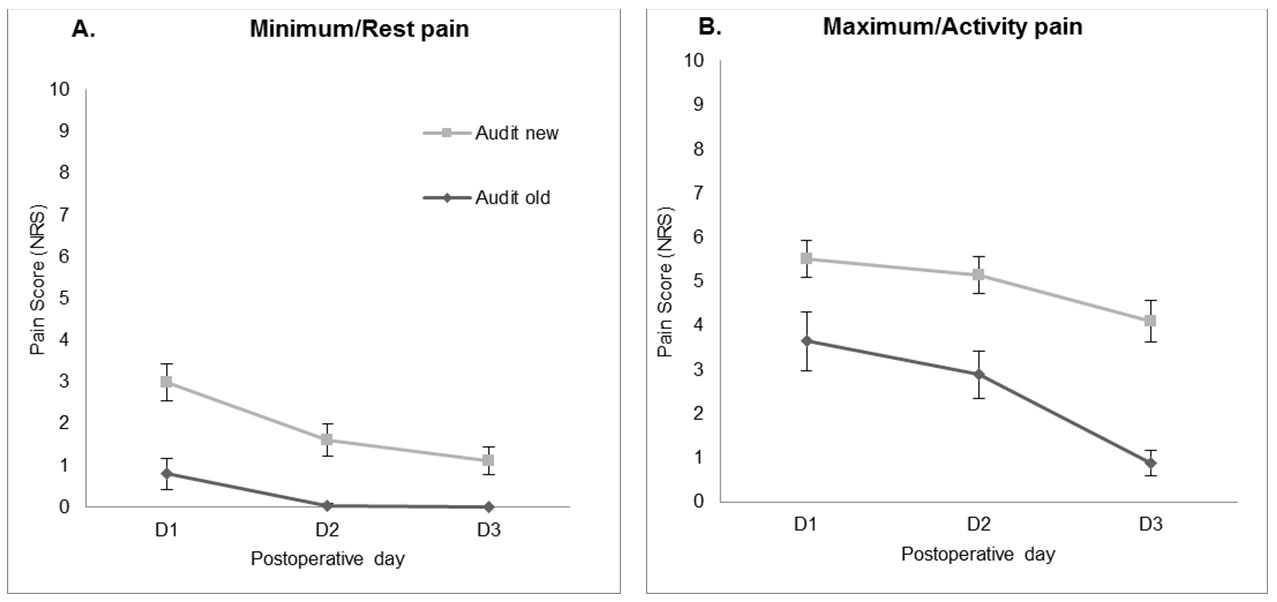
In comparison with the previous audit carried out in 2016, no improvement was noted in the percentage of patients reporting unacceptable level (NRS ≥ 4 at rest) of pain (POD1 38.8% vs 39.13%, POD2 17.8% vs 39.13%, POD3 8.9% vs 3.6%).
The minimum pain scores at rest (1.655 CI 1.061–2.249) and the maximum pain scores with activity (2.592 CI 1.664–3.520) were significantly increased in the current compared to previous audit (both p < 0.001) (Figure 2).
Factors predicting postoperative pain
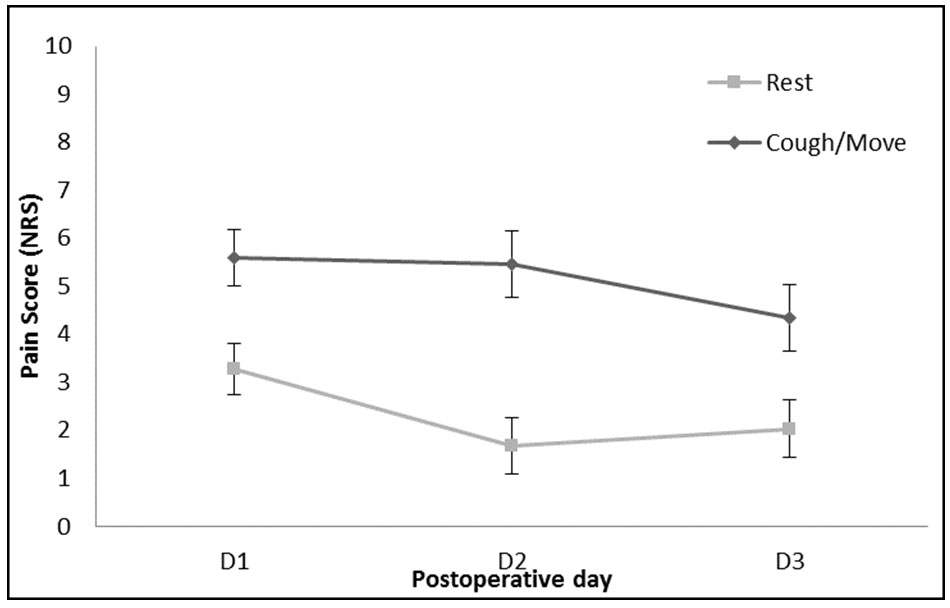
In univariate analysis conducted on NRS at rest, there was a significant effect of postoperative day (p < 0.001), where rest pain was worse on postoperative day 1 than postoperative day 2 (1.267, CI 2.11–0.43, p = 0.003) and worse on postoperative day 3 than postoperative day 2 (1.871, CI 2.81–0.93, p = 0.003) (Figure 3); overall showing that pain is the strongest on POD1. In the univariate analysis conducted on coughing or moving pain scores, there was a significant effect of postoperative day (p < 0.001), where action pain was worse on POD1 than POD3 (1.267, CI 2.11–0.43, p = 0.003). NRS was not significantly less on POD2 compared to POD1 (p = 0.481).
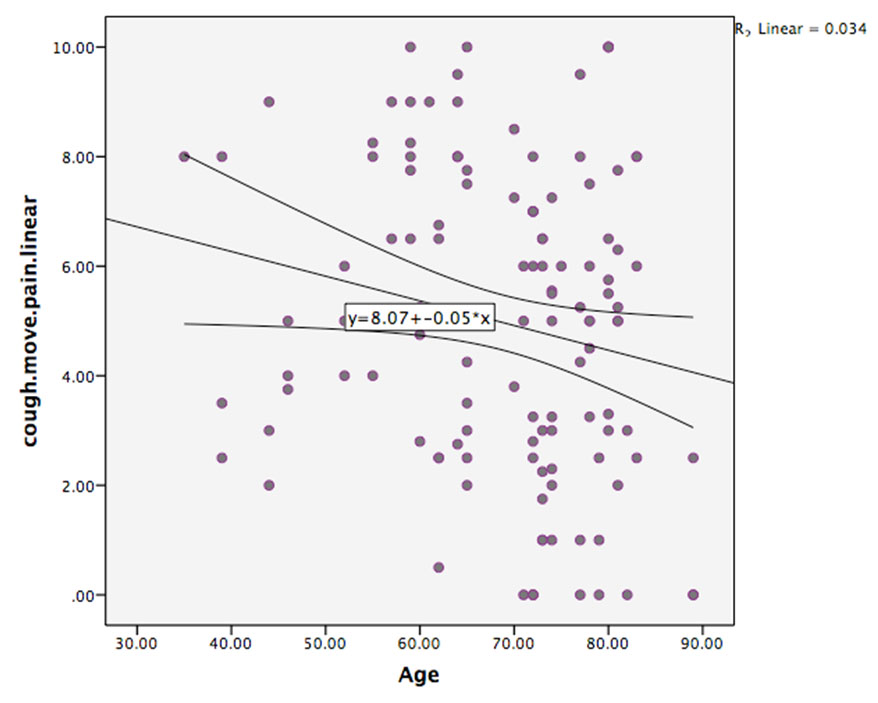
There was no significant effect of age on rest pain. However, there was a trend toward a significant effect of age (p = 0.068) on pain on coughing or moving, with older patients reporting less pain than younger patients.
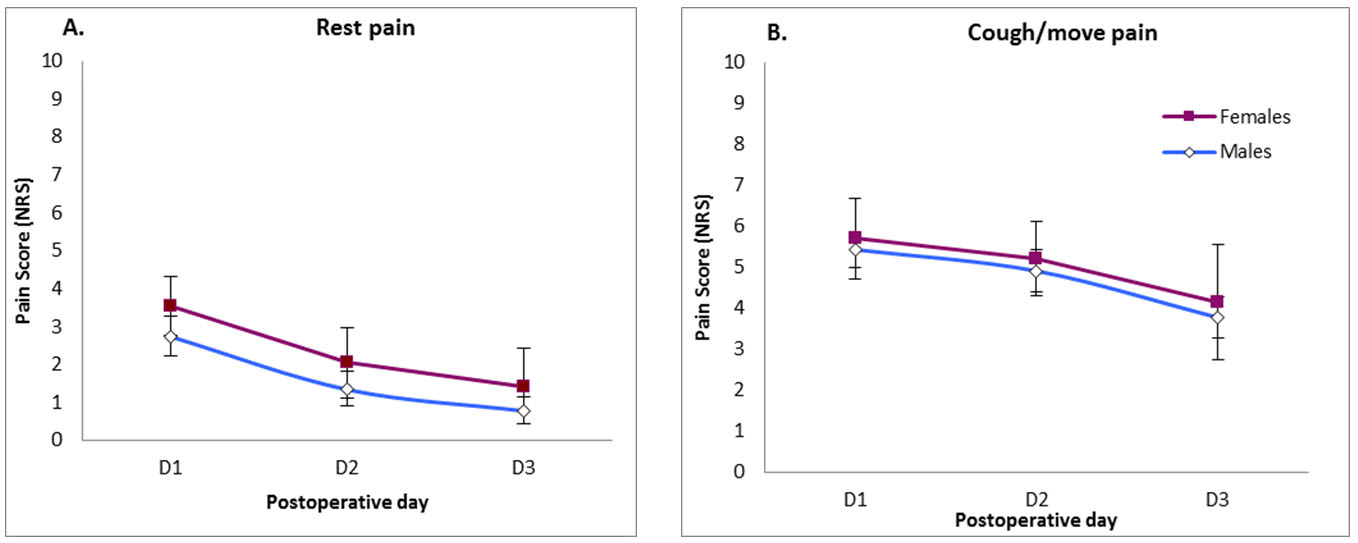
There was a trend toward female patients reporting more pain at rest than males (p = 0.227) (Figure 4), however, no significant difference was noticed with regard to pain on coughing or movement (p = 0.752).
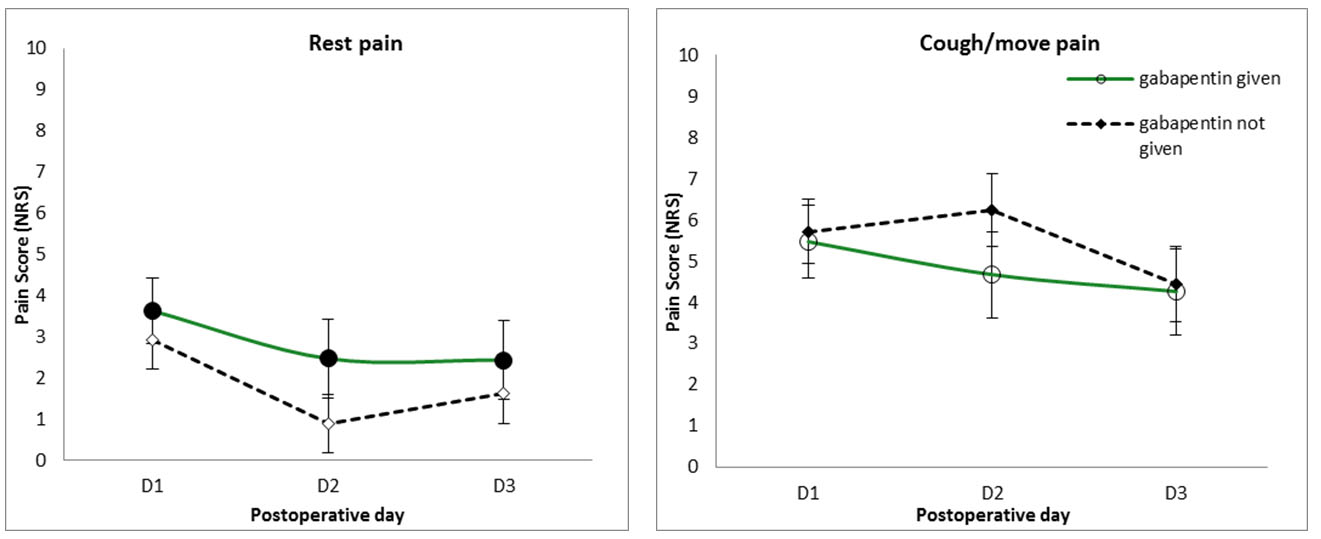
Patients given gabapentin (600 mg) preoperatively tended to have higher pain scores than those who were not given gabapentin; however, this was not statistically significant (p = 0.303). Preoperative gabapentin did not significantly alter pain levels when coughing/moving (p = 0.709) (Figure 5).
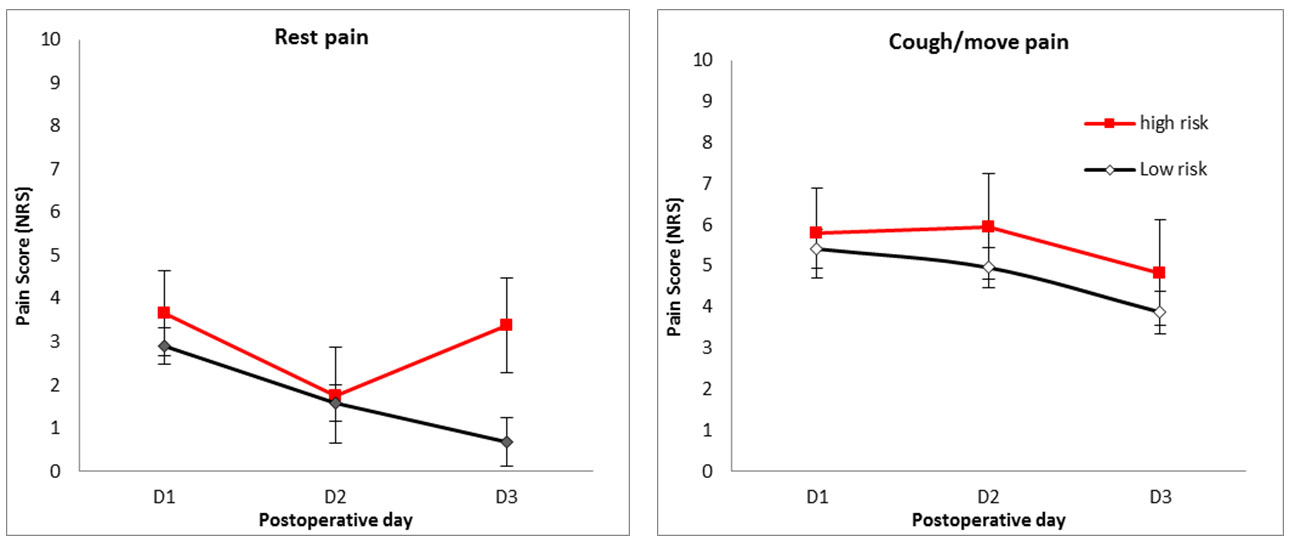
There was no overall significant effect of predicted risk status on rest pain (p = 0.287); however, graphical representation reveals different pattern of postoperative rest pain in high- and low-risk patients, with higher risk patients having higher rest pain on the third postoperative day (Figure 6).
Postoperative day, risk (high or low), gender, and interaction between postoperative day and risk were entered into the multivariate analysis for NRS at rest pain. This analysis confirmed a strong significant main effect of postoperative day, with rest pain on POD1 was greater than on POD3 (2.173, CI 3.18–1.17, p < 0.001). There was an overall significant interaction effect between postoperative day and risk (p = 0.032), driven by the trend for the pain reported by high-risk patients to be greater than that reported by low-risk patients on POD3 (2.14, CI −0.32 to 4.26, p = 0.054).
The factors of postoperative day and age were entered into the multivariate analysis for coughing or moving pain. This analysis again showed a strong significant effect of postoperative day with rest pain: POD1 being greater than on POD3 (2.173, CI 3.18–1.17, p < 0.001), thus demonstrating the finding that pain is strongest on POD1. There was also a trend toward a significant effect of age (p = 0.0518) with older patients reporting lower pain scores (Figure 7).
DISCUSSION
Our audit has shown that despite a change in the protocol no major changes were noted in the reported post-cardiac surgery pain on POD1, POD2, and POD3.
The main reason for lack of improvement of postoperative pain management assessed by NRS scores can be explained with the limited implementation of the new protocol. Hence, we cannot comment on the effectiveness of the updated protocol.
Reasons for failure of protocol implementation are given in the following.
The cardiothoracic pain management protocol was not implemented in the vast majority of patients. Despite recommendations, only half of patients received a stat dose of gabapentin preoperatively. There was no distinction between high-and low-risk patients in terms of their postoperative pain management and the pain team was not called to review or prescribe PCA due to severe pain (NRS ≥ 8). In addition, it was found that the administration of gabapentin had no significant effect on reported pain.
There could be number of reasons for these findings:
- Despite the revisions to the protocol made at the end of 2014 there was a lack of awareness of the protocol revisions and/or lack of appreciation for their importance. The new protocol was circulated via e-mail among the anesthetists. However, cardiac care nurses appeared unaware of some of the guidelines (e.g., to refer high pain scores to the pain team). The implementation of the protocol had missed communication with nurses, acute pain team, pharmacists, and surgeons. This perhaps necessitates a more official introduction of a new pain management regimen, perhaps through multidisciplinary meetings, audit presentations, and formal education sessions. In addition, e-mail circulation to wider number of health professionals involved in cardiac surgery and posters located in anesthetic department and operating theatres, to encourage a multidisciplinary implementation of the new protocol guidelines.
- There were certain instances in which preoperative gabapentin was prescribed but not administered, highlighting the requirement for a team effort to ensure protocol guidelines are followed. Patient factors including refusal or absence may contribute to a small number of failures in preoperative pain administration.
- Differential pain management plan for the high-and low-risk patients requires that risk is assessed preoperatively. Although anesthetists are generally aware of that issue, even before the protocol was introduced, formal preoperative assessment had not been done. The question is whose ownership was the implementation of that particular part of the protocol: anesthetists, pain team, word nurses, or someone else. We concluded that we had to create a questionnaire that can be applicable to other anesthetic preassessments and that it should be structured and easy to perform. Patient self-assessment is also an option for the future. A preoperative workup requires adequate time and appropriate questioning (about previous medication and drug misuse) to stratify patients into high-and low-risk groups.
- Lack of introduction of the use of PCA can be attributed to lack of awareness of cardiac recovery unit (CRU) staff of that part of the protocol, the absence of previous practice of PCA in post-cardiac surgery patients, and the cost and limited availability of PCA in the cardiac recovery unit.
- Although pain scores were measured as ≥8 on 36 occasions in this audit, it is possible that there was a disparity between these scores and those obtained by nurses at the bedside. If patients were not asked to report pain on coughing and moving, then it is likely much lower pain scores would be reported, thus no reporting to the pain team. This highlights a potential issue in current pain assessment measures and suggests healthcare professionals should be encouraged to ask pain levels during these clinically important activities related to risk of postoperative complications. Nurses expressed their lack of awareness in regard to calling the pain team, reinforcing the need to better spread the revised protocol guidelines among staff members.
Factors predicting pain
The strongest factor predicting pain was postoperative day with an overall trend for pain levels to decrease with increasing days after cardiac surgery. This is consistent with previous research [12],[13]. There was a trend for younger patients to report higher levels of pain than older patients, also consistent with previous research [14],[15]. However, in this study, the effect of age only approached significance with pain on coughing/moving, and perhaps this reflects higher activity, more movement or harsher coughing in the younger cohort of patients.
An interesting finding from this analysis is the interaction between risk and postoperative day for pain reported at rest. Differentiating between high- and low-risk patients is a new component of the pain management protocol. This audit suggests this is important distinction to make, thus enabling us to deliver an individualized pain management plan to every patient. It is possible the two groups not only differ in analgesic need but also in the mechanisms responsible for their pain. They therefore may benefit from different combinations of analgesic medication, such as gabapentin, if they experience more neuropathic pain. This is a question for future research.
The pain management protocol introduced the administration of preoperative gabapentin to all cardiac surgery patients. This analysis, however, found no effect of gabapentin on either rest pain or coughing/moving pain on the first three postoperative days. Research into preoperative gabapentin is relatively recent and there is still ambiguity as to its effectiveness [16],[17],[18],[19],[20]. There is suggestion that gabapentin, given its neuromodulating effect, may have a greater effect on chronic postoperative pain, but this analysis cannot answer this question. This analysis here suggests that further support needs to be found for administrating preoperative gabapentin to every cardiac surgery patient to justify its use within the department, for both acute and chronic postoperative pain.
Pain is subjective and patient attitudes, beliefs, and expectations of postoperative pain and recovery after cardiac surgery correlate with reported pain and treatment satisfaction [21]. Preoperative pain sensitivity [22], mood status [23], and psychological factors [24],[25],[26] effect the level or reported pain postoperatively. Even environmental factors including hospital structure, beliefs of hospital personnel, and administrative concerns influence care given and hence pain levels [27]. All these factors might have possibly confounded the reported pain scores in our patients. However, this study was not designed to evaluate this.
Limitations
Pain was not measured at a standardized time after surgery, but every four hours after tracheal extubation, meaning measurements could have been taken after a dose of analgesia, causing less pain and potentially increased sedation at that time. Patients were all seen prior to their surgery to inform them about the audit and to obtain consent, which could have prewarned them about the pain they might experience. When asked about pain on coughing and moving some patients moved or coughed, but not all. Thus, this could influence the pain scores on movement, possibly reducing them for some of patients, therefore, making the pain scores not a true reflection of pain on movement following surgery. Statistical analysis was limited by the lack of preoperative pain scores, and inclusion of small number of cardiac surgery patients. This limits the power with which a regression analysis can be used to explore factors predicting pain. Other limitations include audit type data analysis, retrospective data collection, and being a single centre study.
CONCLUSION
The recommendations that follow from this audit on management of post-cardiac surgery are twofold. First, it is important that there are improved efforts to implement the updated pain management protocol. This involves increasing awareness among the all team members involved in care of cardiac surgical patients. Once the protocol has been successfully implemented then we can measure the effectiveness of this protocol. This study highlights the importance of multimodal and individualized pain management approach. Since pain is highest on POD1 and declines as postoperative day increases there is a scope for structured pain management with stronger analgesic option on day one and slowly wean down.
REFERENCES
1.
Choinière M, Watt-Watson J, Victor JC, et al. Prevalence of and risk factors for persistent postoperative nonanginal pain after cardiac surgery: A 2-year prospective multicentre study. CMAJ 2014;186(7):E213–23. [CrossRef]
[Pubmed]

2.
Milgrom LB, Brooks JA, Qi R, Bunnell K, Wuestfeld S, Beckman D. Pain levels experienced with activities after cardiac surgery. Am J Crit Care 2004;13(2):116–25.
[Pubmed]

3.
White PF, Rawal S, Latham P, et al. Use of a continuous local anesthetic infusion for pain management after median sternotomy. Anesthesiology 2003;99(4):918–23. [CrossRef]
[Pubmed]

4.
Bigeleisen PE, Goehner N. Novel approaches in pain management in cardiac surgery. Curr Opin Anaesthesiol 2015;28(1):89–94. [CrossRef]
[Pubmed]

5.
6.
7.
Quinlan J, Cox F. Acute pain management in patients with drug dependence syndrome. Pain Rep 2017;2(4):e611. [CrossRef]
[Pubmed]

8.
Pain and substance misuse: Improving the patient experience. A consensus statement prepared by The British Pain Society in collaboration with The Royal College of Psychiatrists, The Royal College of General Practitioners and The Advisory Councilon the Misuse of Drugs. [Available at: https://www.britishpainsociety.org/static/uploads/resources/misuse_0307_v13_FINAL.pdf]

9.
Chapman CR, Donaldson G, Davis J, Ericson D, Billharz J. Postoperative pain patterns in chronic pain patients: A pilot study. Pain Med 2009;10(3):481–7. [CrossRef]
[Pubmed]

10.
Garimella V, Cellini C. Postoperative pain control. Clin Colon Rectal Surg 2013;26(3):191–6. [CrossRef]
[Pubmed]

11.
Richebé P, Beaulieu P. Perioperative pain management in the patient treated with opioids: continuing professional development. [Article in French]. Can J Anaesth 2009;56(12):969–81. [CrossRef]
[Pubmed]

12.
Mueller XM, Tinguely F, Tevaearai HT, Revelly JP, Chioléro R, von Segesser LK. Pain location, distribution, and intensity after cardiac surgery. Chest 2000;118(2):391–6. [CrossRef]
[Pubmed]

13.
Chapman CR, Zaslansky R, Donaldson GW, Shinfeld A. Postoperative pain trajectories in cardiac surgery patients. Pain Res Treat 2012;2012:608359. [CrossRef]
[Pubmed]

14.
Sommer M, de Rijke JM, van Kleef M, et al. The prevalence of postoperative pain in a sample of 1490 surgical inpatients. Eur J Anaesthesiol 2008;25(4):267–74. [CrossRef]
[Pubmed]

15.
16.
Ucak A, Onan B, Sen H, Selcuk I, Turan A, Yilmaz AT. The effects of gabapentin on acute and chronic postoperative pain after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2011;25(5):824–9. [CrossRef]
[Pubmed]

17.
Pesonen A, Suojaranta-Ylinen R, Hammarén E, et al. Pregabalin has an opioid-sparing effect in elderly patients after cardiac surgery: A randomized placebocontrolled trial. Br J Anaesth 2011;106(6):873–81. [CrossRef]
[Pubmed]

18.
Menda F, Köner Ö, Sayin M, Ergenoğlu M, Küçükaksu S, Aykaç B. Effects of single-dose gabapentin on postoperative pain and morphine consumption after cardiac surgery. J Cardiothorac Vasc Anesth 2010;24(5):808–13. [CrossRef]
[Pubmed]

19.
Joshi SS, Jagadeesh A. Efficacy of perioperative pregabalin in acute and chronic post-operative pain after off-pump coronary artery bypass surgery: A randomized, double-blind placebo controlled trial. Ann Card Anaesth 2013;16(3):180–5. [CrossRef]
[Pubmed]

20.
Rapchuk I, O’Connell L, Liessmann CD, Cornelissen HR, Fraser JF. Effect of gabapentin on pain after cardiac surgery: A randomised, double-blind, placebo-controlled trial. Anaesth Intensive Care 2010;38(3):445–51 [CrossRef]
[Pubmed]

21.
Leegaard M, Nåden D, Fagermoen MS. Postoperative pain and self-management: Women’s experiences after cardiac surgery. J Adv Nurs 2008;63(5):476–85. [CrossRef]
[Pubmed]

22.
Werner MU, Mjöbo HN, Nielsen PR, Rudin A. Prediction of postoperative pain: A systematic review of predictive experimental pain studies. Anesthesiology 2010;112(6):1494–502. [CrossRef]
[Pubmed]

23.
Morone NE, Weiner DK, Belnap BH, et al. The impact of pain and depression on recovery after coronary artery bypass grafting. Psychosom Med 2010;72(7):620–5. [CrossRef]
[Pubmed]

24.
Khan RS, Skapinakis P, Ahmed K, et al. The association between preoperative pain catastrophizing and postoperative pain intensity in cardiac surgery patients. Pain Med 2012;13(6):820–7. [CrossRef]
[Pubmed]

25.
Lautenbacher S, Huber C, Schöfer D, et al. Attentional and emotional mechanisms related to pain as predictors of chronic postoperative pain: A comparison with other psychological and physiological predictors. Pain 2010;151(3):722–31. [CrossRef]
[Pubmed]

26.
Papaioannou M, Skapinakis P, Damigos D, Mavreas V, Broumas G, Palgimesi A. The role of catastrophizing in the prediction of postoperative pain. Pain Med 2009;10(8):1452–9. [CrossRef]
[Pubmed]

27.
Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth 2008;101(1):17–24. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Zoka Milan - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Amy Rene Gomes - Substantial contributions to conception and design, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Mimi R. Borrelli - Substantial contributions to conception and design, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Gudran Kunst - Substantial contributions to conception and design, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
K Katyayni - Substantial contributions to conception and design, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2020 Zoka Milan et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.