|
Research Articles
Prevalence of preoperative anemia in pediatric surgical patients and its impact on perioperative blood transfusion
1 Consultant, Department of Anaesthesia Great Ormond Street Hospital for Children NHS Foundation Trust London United Kingdom
2 Consultant, Department of Anaesthesia Royal Alexandra Children’s Hospital, Brighton United Kingdom
3 Consultant, Department of Anaesthesia Royal National Orthopaedic Hospital NHS Trust London United Kingdom
4 Consultant, Department of Anaesthesia Great Ormond Street Hospital for Children NHS Foundation Trust London United Kingdom
Address correspondence to:
Ioannis A Ioannou
Department of Anaesthesia, Great Ormond Street Hospital for Children NHS Foundation Trust, London WC1N 3JH,
United Kingdom
Message to Corresponding Author
Article ID: 100019A05II2019
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Ioannou IA, Newton R, Clevenger B, de Beer DAH. Prevalence of preoperative anemia in pediatric surgical patients and its impact on perioperative blood transfusion. Edorium J Anesth 2019;5:100019A05II2019.ABSTRACT
Keywords: Hemoglobin optimization, Patient blood management, Preoperative anemia, Perioperative transfusion
INTRODUCTION
The World Health Organisation’s (WHO) review of the global anemia burden between 1990 and 2010 found the prevalence of anemia to be 32.9%. Over this time period, a reduction in the prevalence of anemia was noted in both sexes for all age groups, with the exception of children under five years of age, where there was an increased prevalence [1]. The incidence of iron deficiency anemia in children from birth to four years in industrialised countries is 19.9% [2], though it is much lower in the United States (0.9 and 4.4% for infants and children, respectively) [3]. With regard to preoperative anemia, a retrospective study of data from the American College of Surgeons National Surgical Quality Improvement Programme (ACS NSQIP) showed that in children aged 1–18 years undergoing noncardiac surgery, the prevalence of anemia was 24.3% [4].
The association of preoperative anemia and adverse outcomes, including mortality, has been shown in a number of adult non-cardiac surgery populations [5],[6]. A recent international consensus statement on the perioperative management of anemia and iron deficiency in adults emphasises the timely recognition of anemia and the introduction of iron therapy as a treatment option in those presenting for surgery with an absolute or functional iron deficiency anemia [7]. The National Institute for Health and Care Excellence (NICE) has also published guidelines to assist in the implementation of pathways for the screening of high-risk patients, and for the diagnosis and treatment of anemia in adults prior to elective major surgery [8].
The diagnosis and treatment of preoperative anemia is a key component in national Patient Blood Management programmes, which aim to reduce unnecessary and inappropriate perioperative blood transfusion [9]. Despite the considerable evidence base in adults, and initiatives to optimize preoperative hemoglobin concentration, there is limited published data on the effects of preoperative anemia, blood transfusion, and outcomes in children. In the same retrospective study of data from the ACS NSQIP, preoperative anemia was shown to be associated with higher odds for inpatient mortality (OR 2.17; 95% CI, 1.48–3.19; p < 0.001). After propensity matching, the presence of anemia was also associated with higher odds of inpatient mortality (OR 1.75; 95% CI, 1.15–2.65; p = 0.004) [4]. In a separate retrospective study of the ACS NSQIP database, of the 2,764 neonates undergoing surgery, a third (32.3%) were found to be anemic. This was noted to be an independent risk factor for mortality [10]. In another study using the same database, 6.987 children who had received a blood transfusion were propensity matched against controls. Children receiving more than 40 mL/kg of red blood cells were found to have a significantly higher 30-day mortality as well as an increased incidence of postoperative infections in a dose dependent manner with increasing transfusion volumes [11].
The aims of the study were to determine the prevalence of preoperative anemia in infants and children presenting for non-cardiac surgery at our institution and to establish whether the presence of preoperative anemia increased the likelihood of perioperative blood transfusion.
MATERIALS AND METHODS
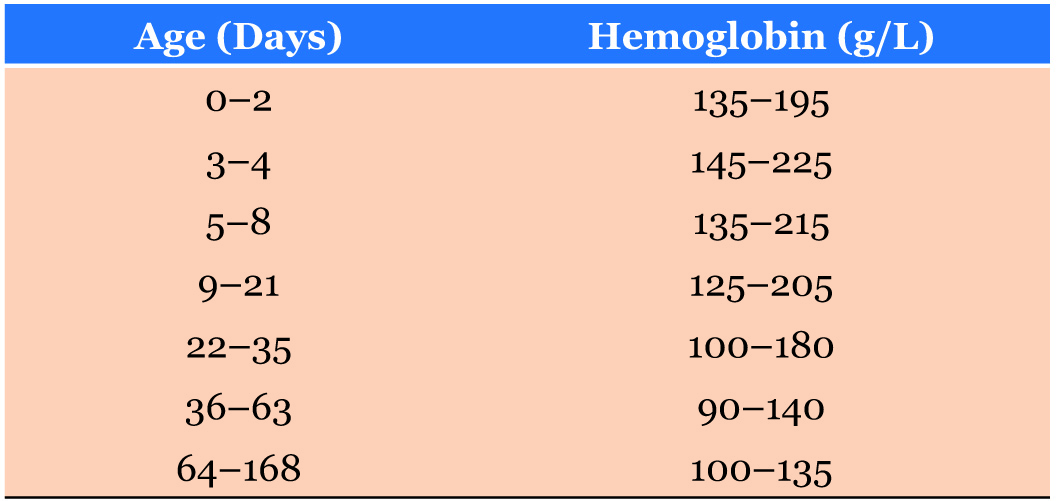
Following institutional research and audit ethical approval, all infants and children [12]. In the absence of a WHO reference range for infants less than six months of age, the institution’s haematology laboratory reference range was used in accordance with international values (Table 1). The incidence of blood transfusion was determined using the institution’s blood transfusion records.
Statistical analysis was performed using GraphPad Prism version 7.00 for Mac OS X, GraphPad software, La Jolla, California, USA, and Microsoft Excel (Microsoft Corp). Continuous variables are expressed as means and standard deviations (SD) or medians and interquartile ranges, and categorical variables as numbers and percentages. A two-sided Fisher’s Exact Test was used to compare the transfusion rates in anemic and nonanemic patients and to analyze anemia and transfusion according to the ASA grade and surgical grading of the patient. A Chi Square Test for Trend was used in the analysis of the severity of anemia and transfusion rates in patients over six months of age. The absence of an anemia severity sub classification in patients less than six months of age resulted in their exclusion from statistical analysis determining associations with receiving a blood transfusion with the severity of anemia.
RESULTS
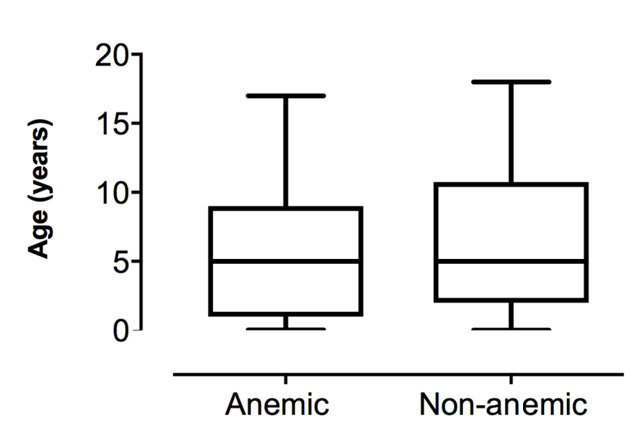
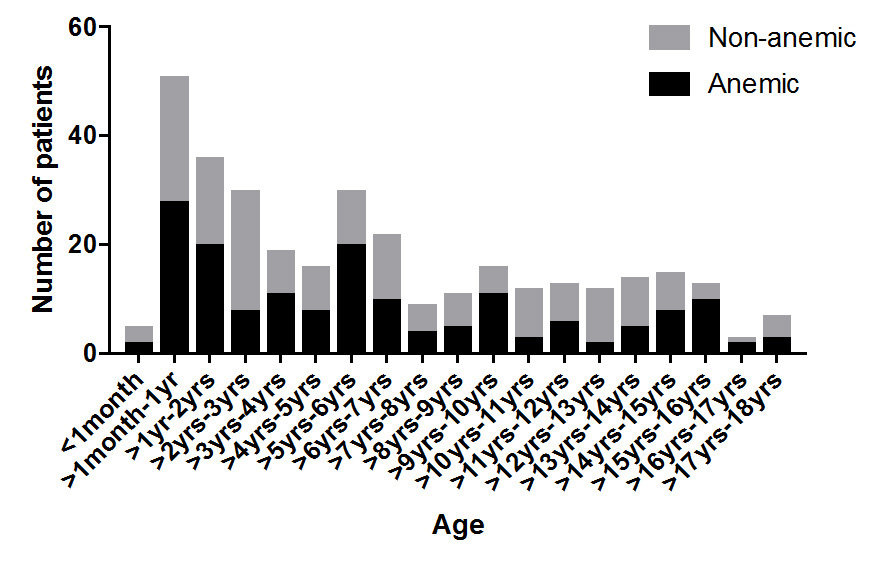
A total of 723 patients underwent elective or emergency non-cardiac surgery during the two-week study period. Almost half (46.2%) of patients had a hemoglobin measurement taken within 28 days of their surgery and were included in the study (n = 334). Of the patients studied, 49.7% (n = 166) were found to have preoperative anemia (hemoglobin concentration below their age specific normal reference range). The mean age was 3.2 years (SD 4.1) and there was a similar distribution of ages in both the anemic and non-anemic groups (Figure 1). The proportion of anemic patients was similar between all age groups (Figure 2). The mean weight for anemic patients was 24.21 kg (SD 2.5) and 24.67 kg (SD 2.4) for non-anemic patients.
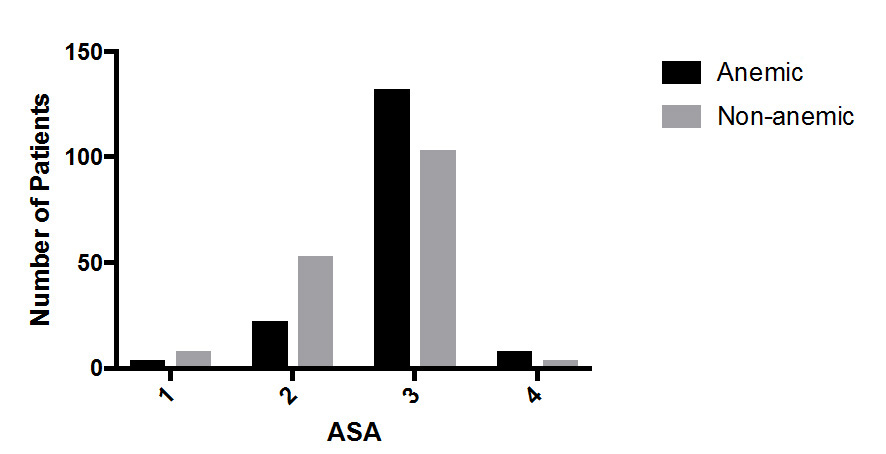
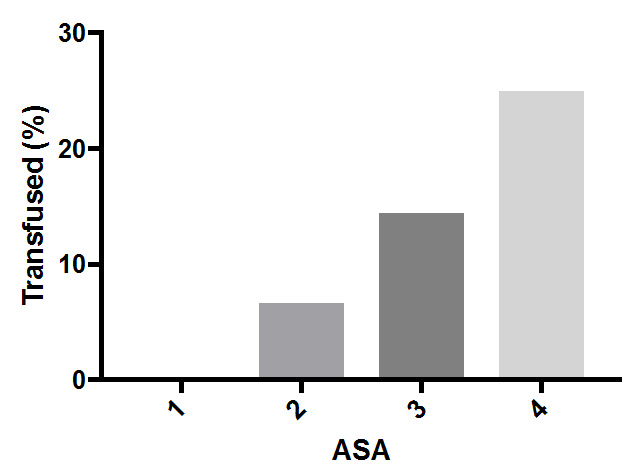
Perioperative blood transfusion occurred in 5.4% of the non-anemic patients compared to 19.9% of anemic patients (OR 4.38; 95% CI, 2.10–9.53; p < 0.0001). The majority of patients were ASA 3–4 (73.95%) with the remainder being ASA 1–2 (26.0%). The prevalence of anemia increased with ASA severity, with over half (56.7%) of the ASA 3–4 patients being anemic, compared to 29.9% of ASA 1–2 patients (OR 3.07; 95% CI, 1.85– 5.27; p < 0.0001) (Figure 3). Blood transfusion occurred more often in anemic than non-anemic patients in both ASA 1–2 patients (11.5% vs 3.3%; OR 3.85; 95% CI 0.73– 22.36; p = 0.15) and ASA 3–4 patients (21.4% vs 6.5%; OR 3.89; 95% CI 1.72–9.76; p = 0.001) (Figure 4).
The majority of cases were scheduled for elective surgery (n = 296) with the remainder being emergency cases (n = 38). All procedures undertaken were stratified into surgical grades [13], with 28.4% being classified as minor, 38.6% intermediate, and 32.9% major or complex (Table 2). The proportion of anemic patients undergoing minor, intermediate and major/ complex surgery was 68.4, 48.4, and 34.2%, respectively. Transfusion occurred more often in anemic patients then non-anemic patients in all three surgical categories, for minor (20.0% vs 10.0%; OR 2.25 95% CI 0.62–7.87; p = 0.38), for intermediate (16.1% vs 3.0%, OR 6.15 95% CI 1.52–28.74; p = 0.01) and for major/complex (18.0% vs 5.5%; OR 3.89 95% CI 1.09–12.43; p = 0.04).
Anemia severity was assessed in all patients older than six months of age in accordance with the WHO anemia severity criteria. The majority of anemic patients were classified as being moderately anemic (n = 110, 75.3%), with the remainder being either mildly or severely anemic (n = 33, 22.6% and n = 3, 2.1%, respectively). As the degree of anemia severity increased, so too did the proportion of patients transfused, however these differences did not reach statistical significance (Chi Square Test of 1.301, p = 0.521).
DISCUSSION
This study has shown that preoperative anemia is common in infants and children presenting for noncardiac surgery at our institution, with anemia being present in almost half of patients in whom a preoperative hemoglobin concentration was available. The majority of patients at our institution were classified as being ASA score 3 or above and the prevalence of anemia was significantly higher in this group. A high proportion of patients undergoing minor procedures were found to be anemic and this represents hematological or oncological patients undergoing lumbar puncture or bone marrow aspiration (Table 2). In an analysis of 51,622 pediatric surgical patients aged 1–18 years from the ACS NSQUIP database, the prevalence of preoperative was found to be 24.3% [4], indicating the prevalence of anemia will vary according to institutional pediatric surgical populations.
This study also demonstrated that children with anemia who underwent surgery were significantly more likely to receive a blood transfusion within 28 days of surgery than those who were not anemic preoperatively. Transfusion rates was significantly higher for anemic patients in both ASA 1-2 and ASA 3-4. The complexity of surgery also influenced the transfusion rates for anemic patients, being significantly more likely to receive blood if undergoing intermediate and major surgery with no significant difference being seen for patients undergoing minor procedures. No significant difference in transfusion rates was identified in patients over six months of age with mild, moderate, or severe anemia although the proportion transfused increased with greater severity of anemia.
Transfusion of allogenic blood is historically the treatment option of choice for perioperative anemia, but this has been shown to increase the risk of adverse outcomes, and should no longer be considered the first response to anemia. Faraoni et al. demonstrated an association between preoperative anemia and an increased risk for in-hospital mortality [4]. This study also demonstrated that both anemic and non-anemic patients who received a blood transfusion had higher mortality rates, with the highest risk seen in anemic patients. In another study it was shown that pediatric patients receiving a blood transfusion had an increased 30-day mortality and there was a correlation between the volume of blood transfused and adverse outcomes [11].
It is clear that preoperative anemia is a potentially modifiable risk factor for both blood transfusion and adverse outcomes after surgery. Diagnosis, investigation, and management should therefore be made a priority. There are NICE and NHS Blood and Transplant guidelines for the implementation of patient blood management programs in adults. Together with international consensus on the management of preoperative anemia, these provide clear guidance with regard to patient selection, diagnostic approaches to iron deficiency anemia, and appropriate treatment options for adults. However, these guidelines cannot easily be applied to infants and children. Patient Blood Management guidelines for neonatal and pediatric patients from the National Blood Authority of Australia recommend that in surgical patients in whom substantial blood loss is anticipated, preoperative anemia and iron deficiency should be identified, evaluated, and managed to minimize blood transfusion [14]. This includes the recommendation to evaluate patients as early as possible in order that surgery can be scheduled to coordinate with the optimization of the patient’s hemoglobin and iron stores. Treatment recommendations include oral iron therapy for those whose surgery is scheduled 6–8 weeks after a diagnosis of iron deficiency.
A challenge to the study of preoperative anemia in pediatrics is the fact that there is little consensus as to how anemia is defined in this patient population, with different definitions being used within the literature. We employed the WHO criteria for hemoglobin using g/L. Faraoni et al. utilized Hct (%) based upon reference values from Nathan and Oski’s. Hematology of Infancy and Childhood to define anemia. Furthermore, the classification of anemia by aetiology, including absolute iron deficiency and functional iron deficiency, also varies between guidelines and within the literature [15].
Anemia in the pediatric population is a global condition with variable geographical etiology and prevalence. Iron deficiency is the most common cause of anemia globally especially in children less than five years of age. Screening for anemia and iron deficiency in adults, as outlined by the international consensus statement on the perioperative management of anemia and iron deficiency, relies initially on serum analysis of hemoglobin concentration, ferritin and transferrin saturation (TSAT), and C-reactive protein. Current recommendations for screening for anemia and iron deficiency in children within the general pediatric population vary according to different professional health bodies. In the United States, the Centres for Disease Control and Prevention recommends screening high-risk patients at 9–12 months of age, six months later and annually thereafter between 2 and 5 years of age [16]. According to the Institute of Medicine, full term infants who are breast fed or who do not receive iron fortified formula milk should be screened at nine months of age [17]. The American Academy of Pediatrics recommends universal screening for anemia at 12 months of age and selective screening at any age in children who are at increased risk for iron deficiency or iron deficiency anemia [18]. In contrast, the US Preventative Task Force have recently published a review of evidence indicating that there is no benefit in screening and treating lowrisk children aged 6–24 months [19]. Despite these varying and often conflicting recommendations, the prevalence of preoperative anemia in infants and children as highlighted in our study suggests that a discussion of the ethics and practicalities of screening in children is warranted. At the same time, it is important that any initiatives to develop a pediatric patient blood management program to facilitate the identification of preoperative anemia and its treatment should include a thorough analysis of appropriate patient selection and consider the implications of introducing a screening program that would necessarily involve a blood test. The use of non-invasive hemoglobin testing devices, may be an alternative way of screening for preoperative anemia, with formal laboratory blood tests being conducted if anemia is detected.
Intravenous iron therapy is recommended for adult patients whose surgery is scheduled within six weeks of diagnosis or who do not respond to oral iron therapy. This includes patients diagnosed with functional iron deficiency where despite body stores of iron being within normal limits, functional utilization is limited by the effects of inflammation or infection. There is on-going research into the efficacy and safety of intravenous iron preoperatively in adult surgical patients [20]. Modern intravenous iron preparations, such as ferric carboxymaltose and iron sucrose, have much improved safety profiles compared to older preparations, such as high molecular weight iron dextrans [21]. Currently iron dextran and ferric carboxymaltose are unlicensed for use in children under 14 years, while iron sucrose is not licensed for use in children, in the United Kingdom [22]. In our institution, ferric carboxymaltose infusions are used in the management of iron deficiency anemia in children with inflammatory bowel disease between the ages of 6–14 years and in patients receiving renal dialysis.
There are a number of limitations with our study, including the relatively short duration of data collection and the unique patient population at a single institution. As a specialist tertiary referral centre, the patients presenting for surgery are generally complex with multiple co-morbidities. Routine measures of hematinics including Ferritin and TSAT are not performed and we were therefore unable to classify the type of anemia present. Although transfusion data were collected prospectively, preoperative blood test results were collected retrospectively, and those patients without preoperative hemoglobin results were excluded as a potentially confounding factor. Additionally, there may have been other reasons besides hemoglobin concentration that influenced the decision to transfuse blood which were not recorded, for example, the early recognition of surgical blood loss. Despite these limitations, our study has highlighted the fact that preoperative anemia is common among infants and children presenting for surgery at our institution and that its presence increases the likelihood of perioperative blood transfusion. With the growing body of evidence of the prevalence of preoperative anemia in children, and its associated increase in the likelihood of receiving a blood transfusion, it is clear that strategies are needed to reduce the associated risk. Providing a robust system of identifying high-risk patients in a timely manner and offering alternatives to blood transfusion so as to optimize hemoglobin concentration is becoming apparent within pediatric perioperative practice. Focus should now concentrate on elucidating the etiology of preoperative anemia in pediatric patients and further research is needed to determine whether the pediatric population would benefit from preoperative screening and optimization with iron therapy to treat functional or absolute iron deficiency anemia.
CONCLUSION
This study adds to the published evidence showing that preoperative anemia is frequent in the neonatal and pediatric surgical population and that this is associated with a significantly higher risk of perioperative blood transfusion. Patients who are anemic having more complex surgery are more likely to receive a blood transfusion then non-anemic patients having similar surgery. Further research is required in order to determine whether preoptimization of anemia in infants and children can reduce the risk of perioperative blood transfusions and improve outcomes after surgery.
REFERENCES
1.
Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014;123(5):615–24 [CrossRef]
[Pubmed]

2.
Levin C, Harpaz S, Muklashi I, et al. Iron deficiency and iron-deficiency anemia in toddlers ages 18 to 36 months: A prospective study. J Pediatr Hematol Oncol 2016;38(3):205–9. [CrossRef]
[Pubmed]

3.
Paoletti G, Bogen DL, Ritchey AK. Severe irondeficiency anemia still an issue in toddlers. Clin Pediatr (Phila) 2014;53(14):1352–8. [CrossRef]
[Pubmed]

4.
Faraoni D, DiNardo JA, Goobie SM. Relationship between preoperative anemia and in-hospital mortality in children undergoing noncardiac surgery. Anesth Analg 2016;123(6):1582–7. [CrossRef]
[Pubmed]

5.
Musallam KM, Tamim HM, Richards T, et al. Preoperative anemia and postoperative outcomes in non-cardiac surgery: A retrospective cohort study. Lancet 2011;378(9800):1396–407. [CrossRef]
[Pubmed]

6.
Baron DM, Hochrieser H, Posch M, et al. Preoperative anemia is associated with poor clinical outcome in non-cardiac surgery patients. Br J Anaesth 2014;113(3):416–23. [CrossRef]
[Pubmed]

7.
Muñoz M, Acheson AG, Auerbach M, et al. International consensus statement on the perioperative management of anemia and iron deficiency. Anaesthesia 2017;72(2):233–47. [CrossRef]
[Pubmed]

8.
National Institute for Health and Care Excellence. Blood Transfusion. NICE Guideline [NG24]. 2015. [Available at: https://www.nice.org.uk/guidance/ng24]

9.
Eichbaum Q, Murphy M, Liu Y, et al. Patient blood management: An international perspective. Anesth Analg 2016;123(6):1574–81 [CrossRef]
[Pubmed]

10.
Goobie SM, Faraoni D, Zurakowski D, DiNardo JA. Association of preoperative anemia with postoperative mortality in Neonates. JAMA Pediatr 2016;170(9):855–62. [CrossRef]
[Pubmed]

11.
Goobie SM, DiNardo JA, Faraoni D. Relationship between transfusion volume and outcomes in children undergoing noncardiac surgery. Transfusion 2016;56(10):2487–94. [CrossRef]
[Pubmed]

12.
13.
National Institute for Health and Care Excellence. Routine preoperative tests for elective surgery. 2016. [Available at: https://www.nice.org.uk/guidance/ng45]

14.
15.
Clevenger B, Richards T. Pre-operative anemia. Anaesthesia 2015;70 Suppl 1:20–8, e6–8. [CrossRef]
[Pubmed]

16.
Recommendations to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep 1998;47(RR-3):1–29.
[Pubmed]

17.
18.
19.
Siu AL; US Preventive Services Task Force. Screening for iron deficiency anemia in young children: USPSTF recommendation statement. Pediatrics 2015;136(4):746–52. [CrossRef]
[Pubmed]

20.
Richards T, Clevenger B, Keidan J, et al. PREVENTT: Preoperative intravenous iron to treat anemia in major surgery: Study protocol for a randomised controlled trial. Trials 2015;16:254. [CrossRef]
[Pubmed]

21.
Auerbach M, Goodnough LT, Shander A. Iron: The new advances in therapy. Best Pract Res Clin Anaesthesiol 2013;27(1):131–40. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Ioannis A Ioannou - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Richard Newton - Acquisition of data, Revising it critically for important intellectual content, Final approval of the version to be published
Ben Clevenger - Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
David A H de Beer - Substantial contributions to conception and design, Analysis of data, Interpretation of data, Drafting the article, Final approval of the version to be published
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2019 Ioannis A Ioannou et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.