|
Original Article
Perioperative risk factors for acute kidney injury following liver resection surgery
1 Consultant Anesthetist, Honorary Senior Lecturer Visiting Professor, King’s College Hospital UK
2 BSc (Hons) Medical Physiology, Kings College London UK
Address correspondence to:
Amy Rene Gomes
BSc (Hons), Kings College London, Hull York Medical School,
UK
Message to Corresponding Author
Article ID: 100017A05ZM2019
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Milan Z, Gomes AR. Perioperative risk factors for acute kidney injury following liver resection surgery. Edorium J Anesth 2019;5:100017A05ZM2019.ABSTRACT
Aim: To investigate and identify potential perioperative risk factors for developing acute kidney injury (AKI) in patients undergoing liver resection.
Methods: This is a retrospective analysis of a single cohort centre of 110 patients undergoing liver resection. The Kidney Disease: Improving Global Outcomes (KDIGO) criteria was used for acute kidney injury (AKI) diagnosis. Preoperative, intraoperative and postoperative variables were recorded. These were then statistically analysed through univariate and multivariate regression analysis.
Results: Acute kidney injury occurred in 17 patients (15.45%). Twelve patients were stage 1 AKI (70.6%), four were reportedly in stage 2 (23.5%) and one patient was in stage 3 (5.9%). Risk factors were identified through univariate regression analysis were ASA status (p=0.023), weight (p=0.001), gender (p=0.01) and baseline serum creatinine (p=0.031). Weight and Americal Society of Anaesthesiologists (ASA) status were also identified in the multivariate regression analysis as being independent predictors for the development of AKI (p=0.014, p=0.021 respectively).
Conclusion: Acute kidney injury stage 1–3 is a common complication following hepatectomy. Risk factors including obesity and presence of comorbidities can contribute to the development of AKI and thus may provide appropriate targets for perioperative treatment.
Keywords: Acute kidney injury, Complications, Liver surgery, Obesity, Postoperative
INTRODUCTION
The development of acute kidney injury (AKI) is a common complication following surgery. Various studies have identified a variable incidence of 3% and 35% AKI after major abdominal surgery [1].
Liver resection surgery differs from all other abdominal surgery. In order to reduce intraoperative bleeding from the liver, the largest organ which comprises 20% of total cardiac output, patients are purposely kept hypovolaemic during the resection phase. Additionally, vascular clamps which are designed to reduce blood loss during liver resection affect haemodynamics. Reduced venous return leads to hypoperfusion of all organs, including the kidneys. Consequently, there is a belief that AKI is more common complication following liver resection surgery than any other abdominal surgery. However, it remains understudied in terms of its incidence, severity, risk factors and impact on the outcome of surgery. The aim of this study was to assess the incidence, severity and risk factors for the development of postoperative AKI in liver resection surgery.
MATERIALS AND METHODS
We performed retrospective data analysis in tertiary hepato-pancreatic-biliary centre from King’s College London which included 110 patients undergoing liver resection over a 4-year period (2012-2016). To assess the incidence of AKI we used the recently introduced ‘Kidney Disease Improving Global Outcomes’ (KDIGO) criteria [2].
The demographic data that was obtained included age, weight, gender, American Society of Anaesthesiologists (ASA) physical status, presence of hypertension and diabetes. Baseline laboratory values included serum creatinine (SCr), estimated Glomerular Filtration Rate (eGFR), aspartate transaminase (AST), albumin and bilirubin. They were measured preoperatively and on day one of postoperative treatment. SCr was measured postoperatively for five days to enable the identification of AKI using the KDIGO criteria.
Surgical data included type of surgery (morphology and open vs. laparoscopic), median duration of surgery and anaesthesia. Perioperative data recorded included epidural vs. intravenous analgesia, median lowest intraoperative blood pressure (BP) and duration, vasoconstrictors and inotropes that were administered intraoperatively, either as infusion or bolus (noradrenaline (NA), phenylephrine, metaraminol, ephedrine) and the duration of administration. Fluid administration, including the volume and whether it would be crystalloid or colloid, plus blood transfusion was recorded. Postoperative renal function data, length of hospital stay (LOS) and mortality were also observed.
Statistical analysis
Continuous variables are expressed as median (25th and 75th percentiles). Categorical variables were summarised as frequency (percentage). Student two sample t-test was used to compare both groups with all the variables.
The primary endpoint (the development of AKI) was compared between the two groups using univariate and multivariate regression analysis. Based on their potential association with the development of AKI, the following factors were selected: age, weight, female sex, baseline SCr, baseline eGFR, baseline serum albumin, baseline AST, and baseline bilirubin concentration, alongside the duration of anaesthesia, duration of hypotension, thoracic epidural and ASA status. To avoid multicollinearity, two or more highly correlated independent predictor values (r > 0.8) were not included in the regression analysis in concert.
All tests were two-sided, and we defined statistical significance at the level of p<0.05. Statistical analyses were conducted using excel version 2016 with ToolPak analysis.
RESULTS
Total 110 patients who underwent liver resection were identified for this study. It was observed that AKI occurred in 17 patients (15.45%). Total 12 patients were stage 1 AKI (70.6%), 4 were stage 2 (23.5%) and 1 patient was stage 3 (5.9%).
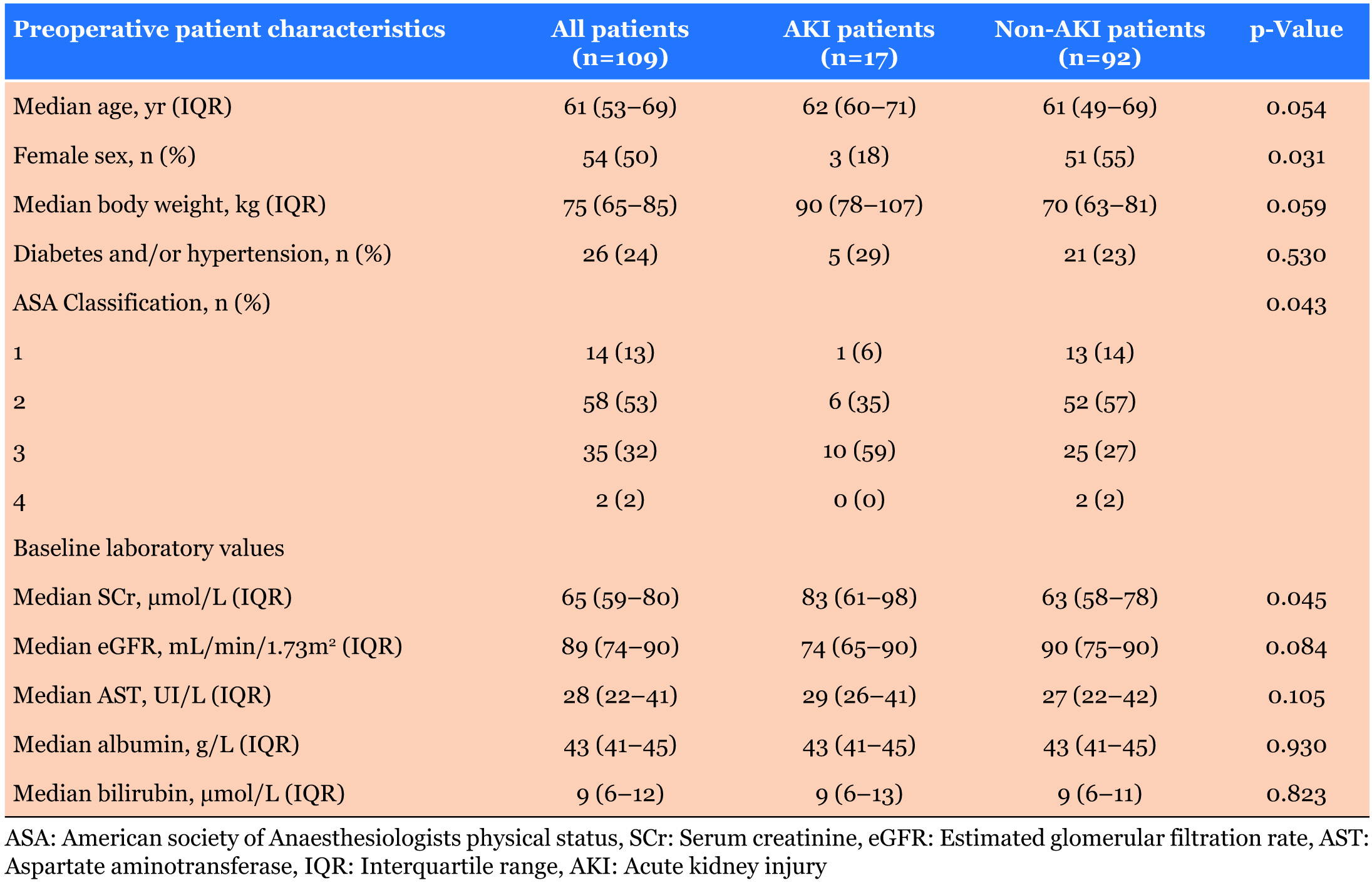
Demographic data are presented in Table 1. There were significantly more male patients in the group that developed AKI, and they had significantly higher ASA score and elevated baseline SCr level. Body weight (p=0.059) and age (p=0.054) were not statistically different between the two groups.
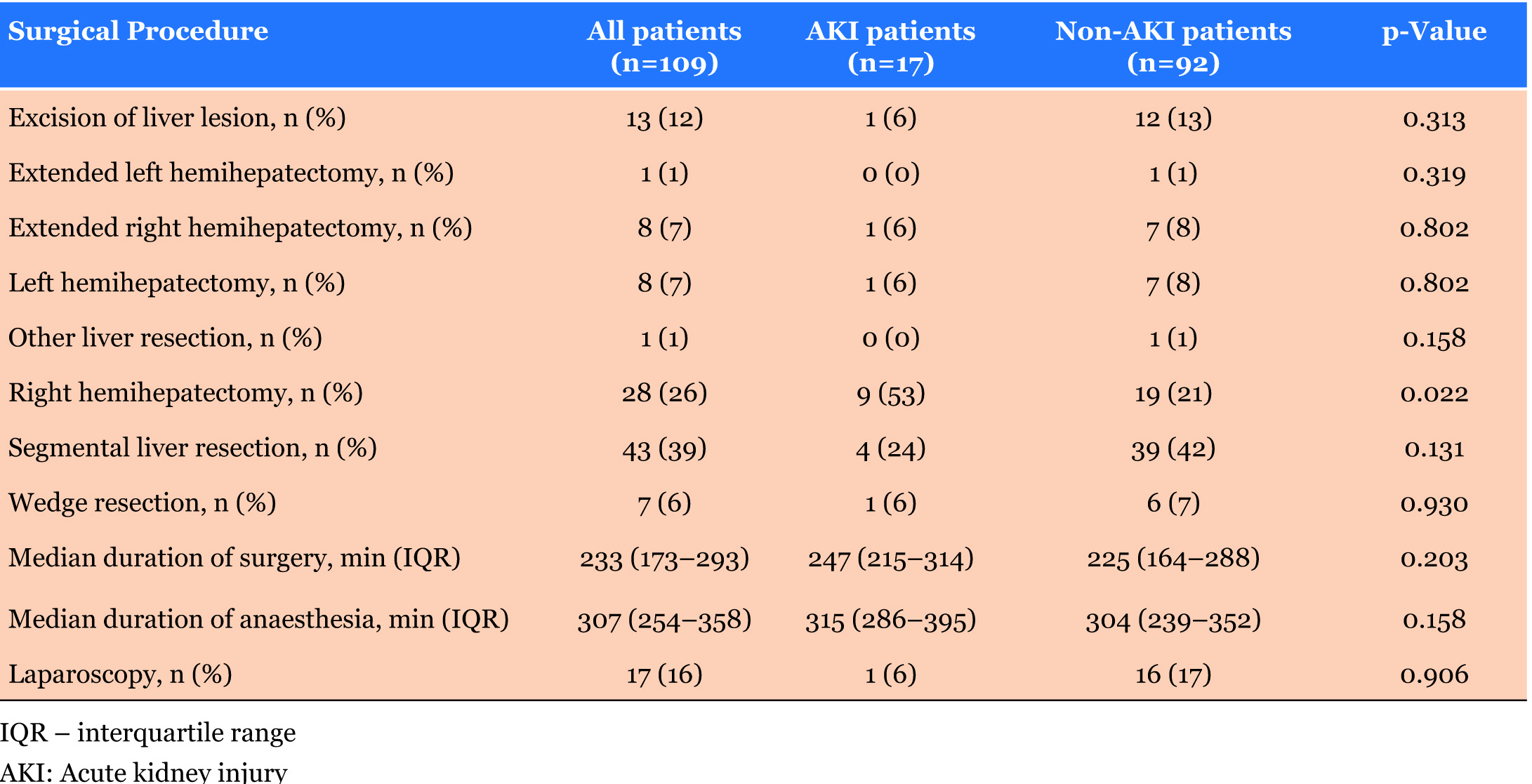
We divided surgical liver resections into eight main categories. Only patients who underwent right hemihepatectomy had significantly higher incidence of AKI. Only 16% of all operations were laparoscopic and we did not find the difference of AKI incidence between laparoscopic and open surgery significant (Table 2).
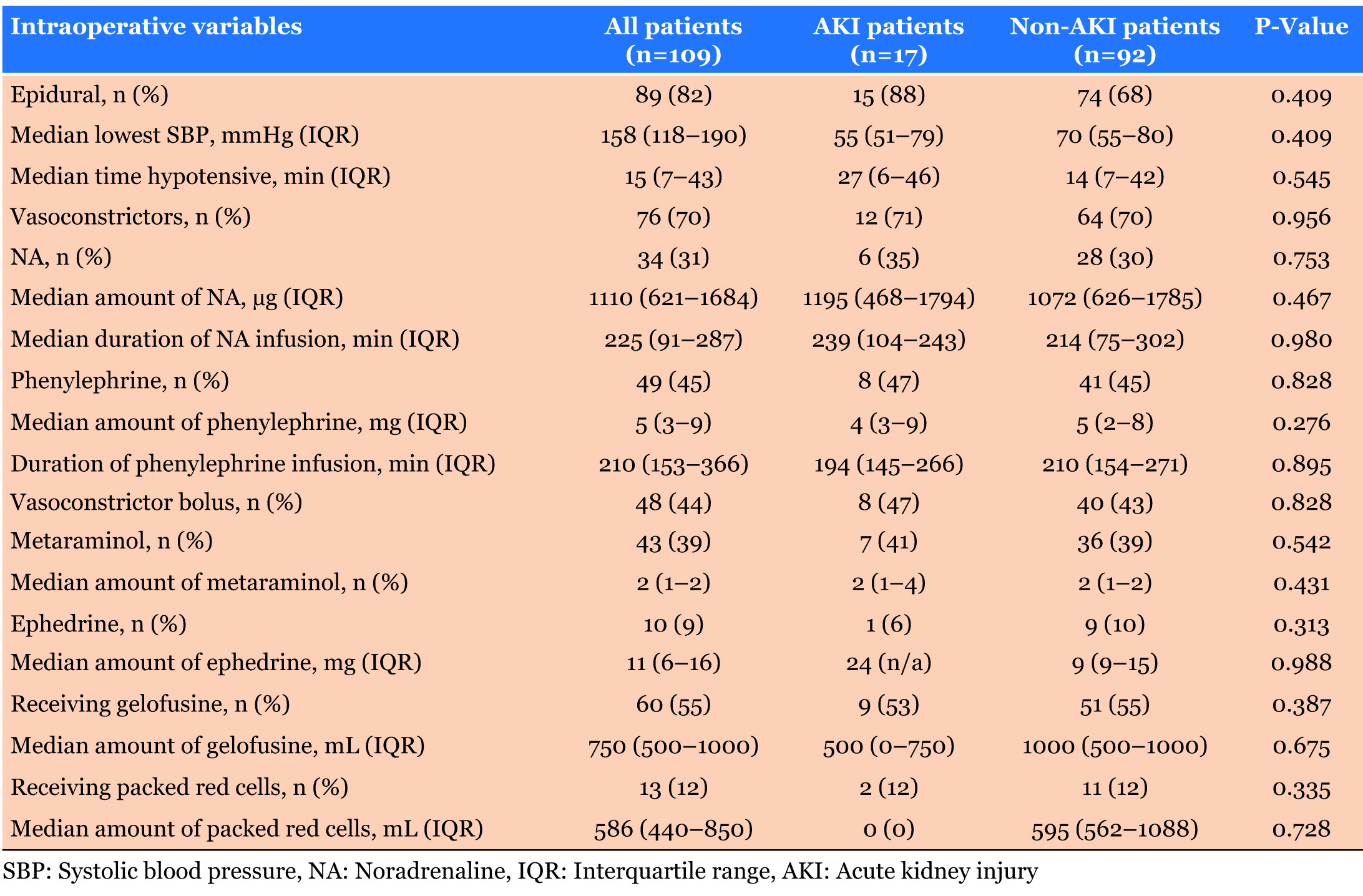
Intraoperatively, no significant differences were seen between AKI and non-AKI patients in many parameters including lowest systolic BP (p=0.409), duration of NA infusion (p=0.980) and administered amount of metaraminol (p=0.431). Duration of surgery and anaesthesia did not differ significantly between AKI and non-AKI patients, alongside the use of epidural analgesia (Table 3).
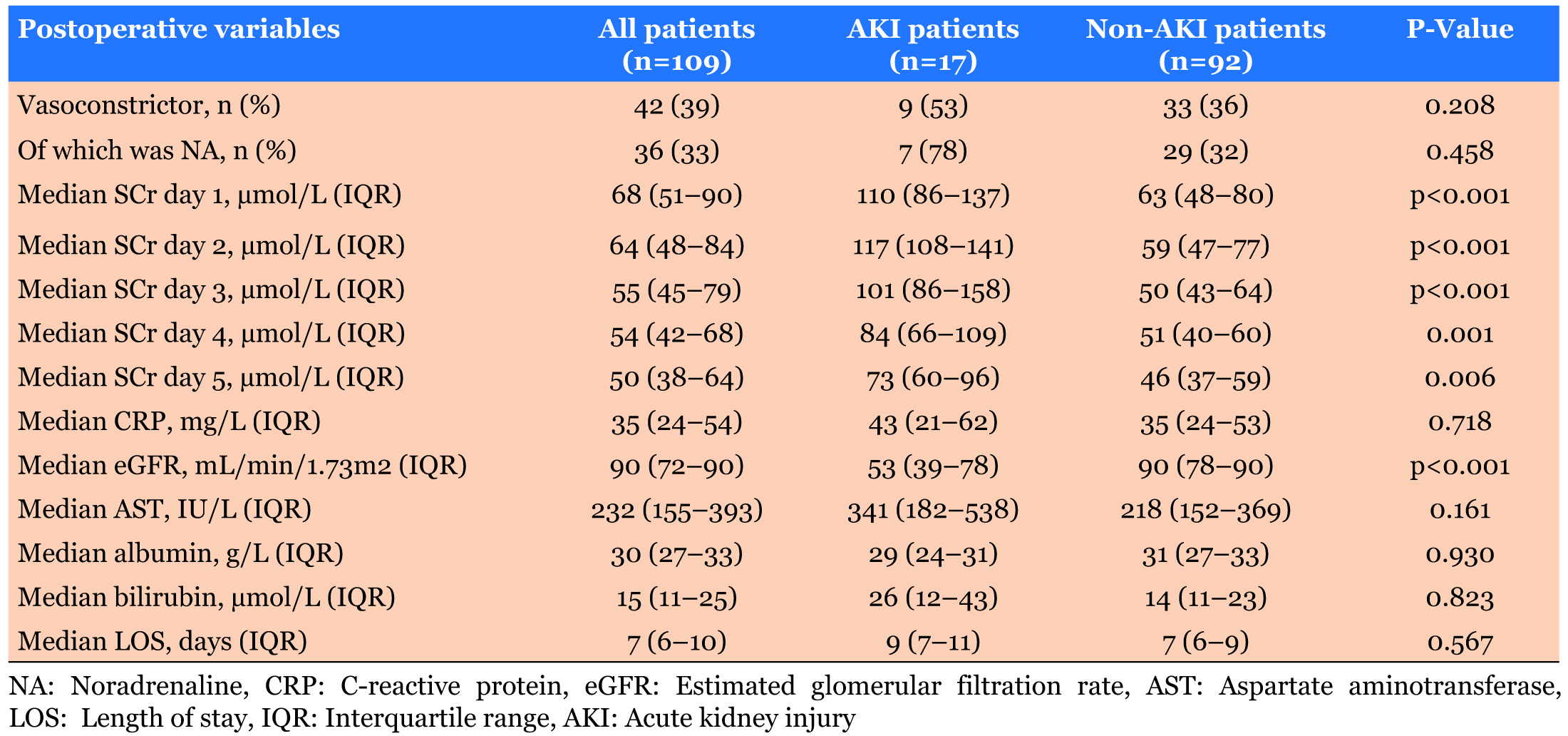
As shown in Table 4, all five postoperative days of SCr and eGFR was significantly lower in non-AKI patients (p=0.001). Patients length of stay was not significantly different between the AKI and non-AKI groups (p=0.567).
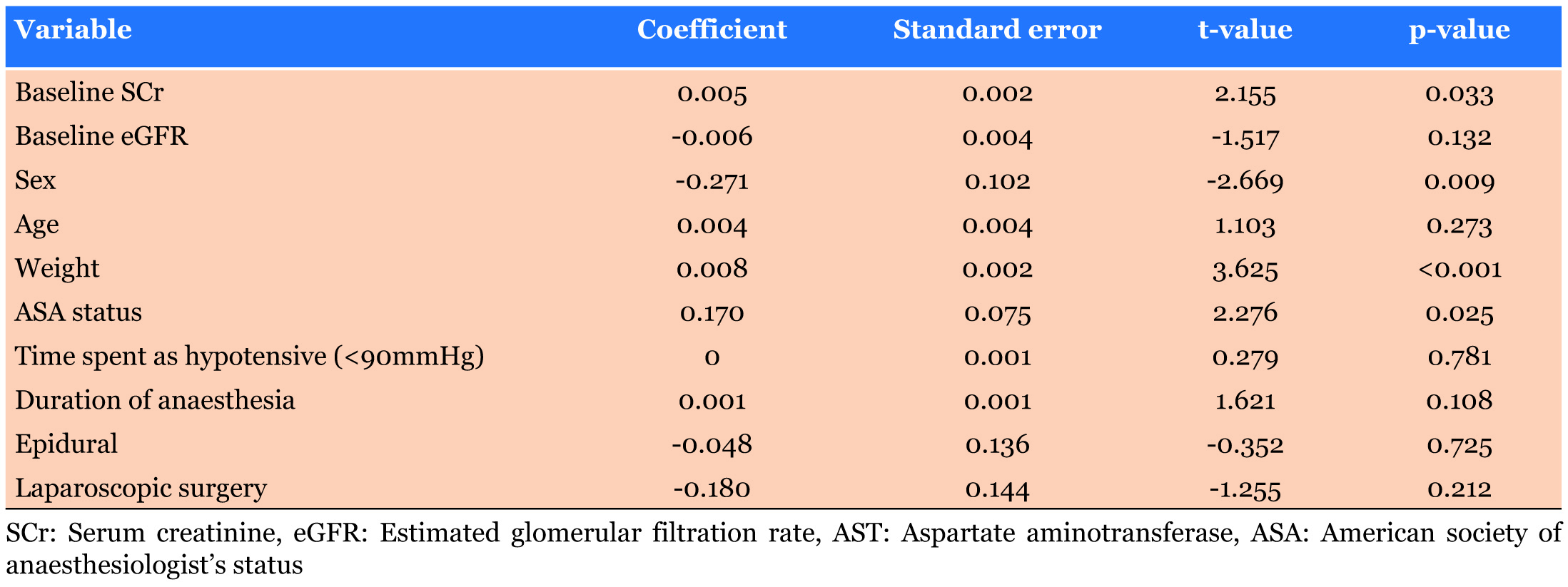
Risk factors identified through univariate regression analysis (Table 5) were ASA status (p=0.025), weight (p<0.001), male gender (p=0.009) and baseline serum creatinine (p=0.033).
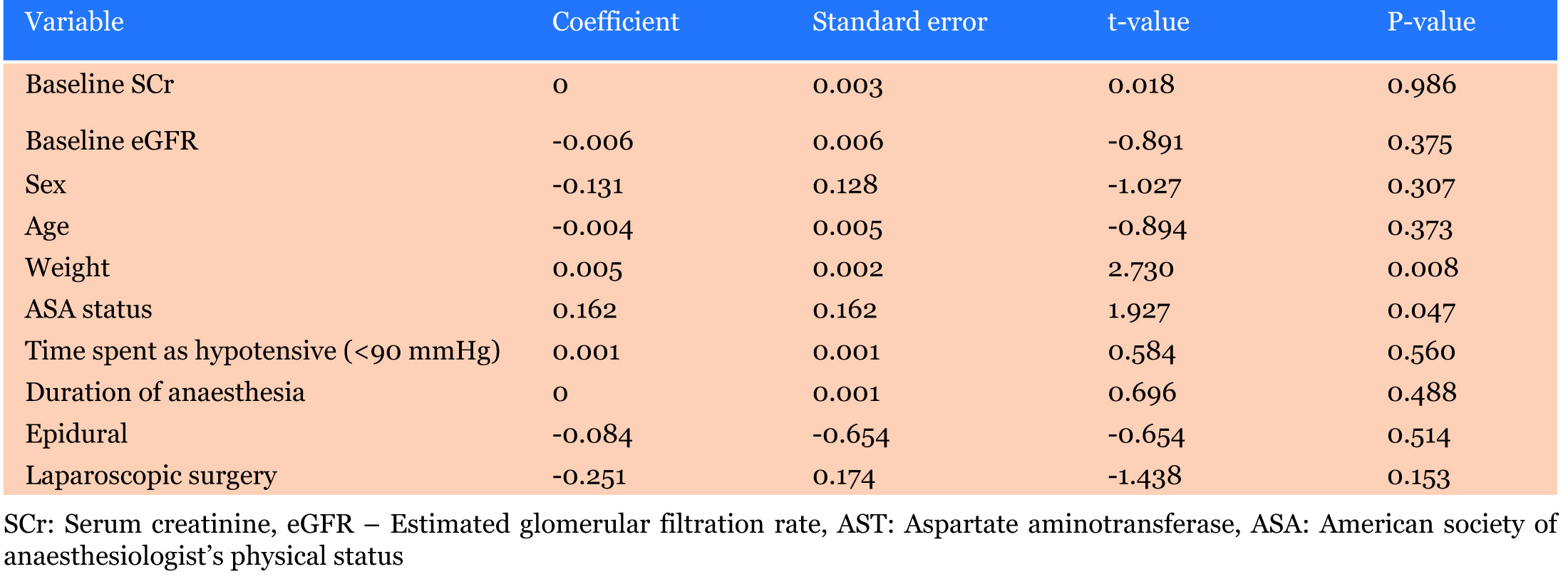
Weight and ASA status were also identified in the multivariate regression analysis as being independent predictors for the development of AKI (p=0.008, p=0.047 respectively) as seen in Table 6.
DISCUSSION
This study assessed the risk factors perioperatively that may be associated with the development of AKI following liver resection surgery. This involved identifying potential risk factors for postoperative AKI in 110 patients undergoing liver resection surgery during the period of 30 August 2012 to 27 July 2016.
Postoperative AKI, defined using the KDIGO criteria, occurred in 17 patients undergoing liver resection surgery (15.45%). Once these patients were identified as having AKI, they were then staged for severity. 12 patients were stage 1 (70.6%), 4 patients were stage 2 (23.5%) and 1 patient was stage 3 (5.9%).
The variables that were associated with the outcome of postoperative AKI in the univariate regression analysis were weight, being of the male sex, preoperative SCrand ASA status. The variables that were identified in the multivariate regression model to be independently associated with the outcome of postoperative AKI were weight and ASA status.
The incidence of postoperative AKI in this present study is similar to that of the study carried out by Slakamenac et al. in 2009 (86/569 patients, 15.1%) and in their further study in 2013 (82/549 patients, 15%), where postoperative AKI was defined using the risk, injury, failure, loss of kidney function and end-stage kidney disease (RIFLE) criteria within 48 hours following surgery [3],[4]. They identified blood transfusions, hepaticojejunostomy, and oliguria to be the strongest predictors for AKI following liver resection. In our study, blood transfusions were not statistically different between AKI and non-AKI patients. In addition, oliguria/urine output was not used here due to inconsistent recordings of urine output on patient charts.
A retrospective study was investigated perioperative risk factors for AKI after liver resection in 642 patients [5]. AKI occurred in 78 patients (12.1%) using the Acute Kidney Injury Network (AKIN) criterion. The risk factors identified that were independently associated with postoperative AKI were intraoperative red blood cell (RBC) transfusion, preoperative hypertension and preoperative eGFR, somewhat different to this study. A similar incidence was also identified by Ishikawa et al. They found that AKI occurred in 27/228 patients (11.8%), where AKI patients had significantly lower preoperative Hb concentrations, higher SCr, lower eGFR and lower serum albumin concentrations [6]. However, in another study investigating the incidence and risk factors for AKI, the incidence of such differed from the above with only 10 out of 131 patients (7.6%) developing AKI, using the AKIN criterion [7], with fluid balance during surgery, urine neutrophil gelatinase-associated lipocalin (NGAL) and liver transplantation being independently associated with a poor six months renal outcome. Similarly, Kambakamba et al., identified an incidence of 8.2%, with 94/1153 patients developing AKI after major liver resection alongside Moon et al., who identified an incidence of 6.6% (77/1173 patients) using the KDIGO criteria [8],[9]. Kambakamba et al. also found that CKD and major liver resection were significantly different between AKI and non-AKI patients, similar to our findings [8]. Moon et al. also identified being of the male sex, elevated preoperative SCr and increased weight to be associated with the development of AKI post liver resection [9]. A greater incidence of AKI was found by Correa- Gallego et al. with 338/2116 (16%) patients developing postoperative AKI [10]. In addition, they similarly found larger resections and lower eGFR preoperatively to be statistically significant between the two groups of patients. Similar incidence was seen in the study carried out by Lim et al. who identified 67/457 (15%) patients that developed AKI following liver resection [4]. This study also identified major hepatectomy, increased weight and elevated baseline SCr to be statistically significant between the two groups of patients. In addition, they identified surgical approach as being significant, however we did not. A retrospective study involving 424 liver transplant recipients and the incidence of postoperative AKI identified similar predisposing factors to those studies mentioned above and this current study [11]. The factors identified to be associated with the development of postoperative AKI were weight, liver disease severity, female sex, pre-existing diabetes mellitus and the number of blood or fresh frozen plasma units during surgery that were transfused.
An issue, however, with comparing the incidence of AKI in the liver resection surgical setting is the use of different AKI definitions. For example, Slakamenac et al. defined AKI using the RIFLE criteria [12]. Tomozawa et al., also used a different criterion: AKIN [5]. In our study, we defined AKI using the KDIGO criteria and this was used over a 5 day postoperative period rather than within a 48 hour period post-surgery. The different definitions of AKI make the comparison of incidence and prevalence of the development of AKI among different studies increasingly difficult. Several risk scores and prediction models for AKI exist for cardiac surgery. However, predictors in these models are generally specific to their particular specialities, rendering some inapplicable to a liver resection surgery setting.
Various risk factors for AKI have been discussed as follow:
Obesity
Increased weight and obesity have profound effects on morbidity and mortality. It has been identified in many studies that the risk of developing postoperative AKI increases with increasing body mass index (BMI). In a retrospective analysis, carried out by Glance et al. 351,000 patients from the American College of Surgeons National Surgical Quality Improvement database were investigated and it was found that there was a two- to three-fold increased risk of postoperative AKI in the obese population [13]. When compared to normal weight patients, this risk was around seven times higher if the obese patients also had metabolic syndrome [13]. In addition, a recent study by Shashaty et al. showed AKI to be correlated with abdominal fat, measured by computed tomography, in ICU patients [14]. Negative effects of a higher BMI on the development of AKI following liver resection was also identified by Moon et al. [9]. Heavier/ obese patients often possess comorbidities such as hypertension, diabetes, and coronary artery disease. This can further complicate perioperative care.
Obesity-related glomerulopathy is common in over weight patients and are characterised by increased GFR, glomerulomegaly with/out evidence of focal segmental glomerulosclerosis, and increased renal plasma flow [15]. These changes put these patients at higher risks of developing postoperative AKI due to preexisting glomerular pathologic conditions and lack of its recognition.
Female gender
This study identified being of the female sex to be statistically significant to the development of postoperative AKI following liver resection; in that being female reduced the odds of outcome. Several studies have identified that the female sex is associated with lower incidence of postoperative AKI after noncardiac surgery [16],[17]. This finding may be suggestive of the beneficial effects of oestrogens in the cardiovascular system and in renal disease - in that they exert an immunoprotective effect, whilst testosterone is thought to exert an immunosuppressive effect [18]. This is supported by some studies that have found that the progression of CKD is typically slower in women compared to men [19],[20]. In addition, male sex is seen to double the propensity for developing post-resectional liver failure and postresectional morbidity [21],[22].
Contradictory to this, most of the patients undergoing liver resection surgery in this study are older and the women are most likely to be postmenopausal meaning that their oestrogen levels are similar to that of a male patient. In addition, it has been repeatedly identified that being a woman increases the risk of developing AKI following both non-cardiac and cardiac surgery. For example, a study carried out by Hilmi et al. which investigated the development of AKI following liver transplant surgery, identified that being of the female sex increased the risk of developing postoperative AKI [11]. In addition, being of the female gender increased the patient’s risk of developing postoperative AKI following cardiac surgery [23]. The increased risk of developing AKI with being female is thought to be related to the overestimation of preoperative renal function in the context of lower muscle mass [24].
Elevated serum creatinine (SCr)
Elevated SCr preoperatively was identified as being a risk factor for the development of AKI in the univariate regression analysis in this study.
Higher baseline SCr is usually reflective of a reduced GFR and the possible progression of CKD. However, SCr is also altered if the patient is dehydrated, hypovolemic or has eaten a large amount of meat.
Baseline SCr is reflective of the patient’s pre-morbid kidney function and thus if it is elevated this may be suggestive of renal dysfunction and the presence of CKD. Clear knowledge of preoperative kidney function is essential to distinguish AKI from CKD. Grading AKI severity requires known baseline function; as a reference for identifying if recovery has finished; and as a way for stratifying patients with and without underlying CKD [25]. It is intuitive that patients who develop postoperative AKI but who also have underlying CKD may present and behave differently due to the complex interaction that occurs among AKI and CKD, in that CKD predisposes patients to an increased risk of AKI, whereas, regardless of renal baseline function, patients with AKI are more likely to suffer from post-AKI CKD [26],[27]. Contrary to this is the finding that a patient in this study developed AKI preoperatively and was admitted to liver ICU for inotropic support and CVVH but was identified as a non-AKI patient postoperatively. This may reflect the benefit of using preoperative optimisation to preserve and improve kidney function. However, this patient did have pre-existing CKD and had a greater length of stay in hospital (26 days) supporting the hypothesis that CKD can influence the development of complications, pre-, intra- and postoperatively, in patients undergoing liver resection surgery.
Hemihepatectomy
In this study, right hemihepatectomy was identified as being significantly different between AKI and non-AKI patients. Right hemihepatectomy involves the resection of V-VIII segments of which is a large part of liver being that only 1/7th of the liver is left. Death and regeneration of the remaining hepatocytes occurs following resection of liver mass [28]. It needs to be noted that in this study, KCH is a specialist liver transplant centre and surgeons are highly experienced. Physiologically, regeneration is greater than the death of hepatocytes and liver mass and function is usually restored rapidly through increased metabolic demand. There is evidence that liver mass restorate of up to 74% of the initial volume during the first ten days after right hemihepatectomy [29]. However, the ability of the liver to regenerate after resection is dependent on the quantity and quality of the residual liver parenchyma and a variety of intraoperative and postoperative factors, for example ischaemia-reperfusion injury and hepatic parenchymal congestion. These factors can also contribute to an increased risk for developing AKI.
Bredt & Peres investigated AKI risk factors after partial hepatectomy and identified that the extent of liver resection was independently associated with the development of postoperative AKI [30]. They identified that patients who developed postoperative AKI underwent more extensive surgeries, and especially, had significantly higher rates of RBC transfusion and hemodynamic instability during liver resection than non-AKI patient, 28.8% vs 8.5%, 31.2% vs 7.1% respectively (p<0.001 for both variables) [30]. Furthermore, in the analysis of a large series of liver resection surgeries, major liver resection was an identified risk factor for postoperative AKI [4]. This may reflect that minor hepatectomies can frequently be carried out safely without measures such as fluid restriction and thus have a lower impact on hemodynamics which may preserve renal autoregulation and maintain renal perfusion.
Consideration must be given to the surgical technique planned and thus the implications the procedure may have on renal function. Kidneys can become vulnerable if the procedure decreases renal perfusion, either through global reduction in perfusion pressure (as may occur in pneumoperitoneum at laparoscopy) or regional reduction in blood flow (e.g. during cross-clamping). The majority of patients who developed AKI underwent open surgery (n=16, 94.12%), however there was no significant difference found between laparoscopic surgery in AKI and non-AKI patients in this study (p=0.906).
In contrast, an observational study by Moon et al. identified that the incidence of postoperative AKI was lower after laparoscopic liver resection compared to open liver resection: 1.8% vs 6.3%, p=0.026 [9]. In previous studies it has been suggested that laparoscopic surgery induces less systemic inflammation when compared to open surgery, as supported by inflammatory markers such as white blood cell (WBC) count, interleukin-6 (IL-6) and CRP level being significantly reduced in laparoscopic procedures vs open surgery [31],[32]. It is thus conceivable that the use of laparoscopic surgery and its immunological benefit may facilitate the prevention of the development of postoperative AKI following liver resection. However, laparoscopic techniques usually involve longer periods of pneumoperitoneum and therefore increase the risk of developing AKI. The potential risk of bleeding during laparoscopic parenchymal dissection could contribute to hypovolemia and intraoperative hypotension and thus increase patients’ susceptibility to develop AKI. This can be compensated for by adequate volume status and intraabdominal pressure maintenance.
We had a small number of laparoscopic procedures and that may cause no statistical significant differences in the development of AKI post liver resection.
Epidural analgesia (EDA) is the standard analgesia for many types of major surgery including liver resection. Liver resection patients are purposely kept hypovolemic in order to reduce blood loss. This is also carried out alongside a low central venous pressure (CVP), which is maintained between 0 and 5 mmHg, through fluid management. EDA commonly induces arterial hypotension because of sympathicolysis and subsequent vasodilation. This combined with LCVP can lower mean arterial pressure (MAP) even further, possibly compromising renal blood flow autoregulation thus contributing to the development of postoperative AKI [8].
Mandatory fluid restriction thus requires the use of vasoactive drugs to maintain MAP; however, this can further contribute to renal hypoperfusion through the constriction of afferent arterioles. Inadequate renal perfusion can induce inflammation, renal hypoxia, and fibrosis, which increases microvascular dysfunction in hemodynamically compromised conditions, i.e. during hepatectomy surgery [33],[34]. In our study, there were no significant differences between the amount or duration of vasoactive drug administration in AKI and non-AKI patients (Table 5). In addition, all patients in this study were treated with tazocin as an antibiotic prophylaxis treatment three days postoperative.
Hypotensive effects of EDA can remain postoperatively and can exacerbate AKI and may require large volumes of intravenous fluid to be administered - another risk factor for AKI and other postoperative complications including pulmonary oedema. Sakowska et al. found that there was a 10-fold increased risk of adverse respiratory events due to large intravenous fluid changes in liver resection patients with EDA [35]. Kambakamba et al. identified EDA as an independent risk factor for AKI following major liver resection [8]. It has also been found that EDA was associated with increased use of intraoperative colloids and catecholamines which have both been previously reported as risk factors for AKI [12].
A common side effect of EDA is sympathetic blockage which usually requires greater intravenous fluid loads or catecholamine administration. This agrees with the theory that EDA, associated with fluid restriction to maintain LCVP, reduces MAP below a threshold that affects and lowers GFR and induces AKI. This is further supported by the well documented relation between the duration and severity of hypotension in patients in the ICU and the risk of AKI [36].
EDA does have it benefits and most studies have confirmed satisfactory analgesia with EDA for liver resection [37]. This is consistent with the finding by Kambakamba et al.who found that there was a 75% reduction in early postoperative opioid administration in EDA patients [8]. However, there is some remaining controversy surrounding the most effective delivery of analgesia during liver resection in that alternate approaches, such as local infiltration combinations with PCA, or bupivacaine intramuscular infusions with EDA, seem hopeful [38],[39].
LIMITATIONS
The findings in this study should be viewed in light of its limitations. As in any observational and retrospective study, the influence of any unmeasured factors on the risk for developing AKI cannot be considered. In addition, it has only highlighted associations between the development of AKI and various risk factors; it has not proved a definite causative relationship, something that limits observational studies. Therefore, data was only extracted on variables for which there is a plausible, biologically causative relationship with the development of AKI.
In addition, the data was derived from a single cohort centre with a small patient sample size, limiting reliability and validity. Data entry errors on anaesthetic charts limited the extent of some of the analysis, including fluid administration and blood and urine losses.
CONCLUSION
There is a compelling evidence for potential risk factors in the development of AKI following liver resection; and as such, with further data collection and analysis, it would be easier to investigate deeper into the physiological causes of AKI following hepatectomy. The identification of perioperative risk factors would allow for the refinement of existing care pathways and the development of new ones where current approaches are not fit for purpose. As such, this would enable the delivery of the best and most appropriate preoperative, intraopertive and postoperative care to meet the needs of patients who are at a greater risk of developing AKI in the liver resection surgical setting.
REFERENCES
1.
Gameiro J, Fonseca JA, Neves M, Jorge S, Lopes JA. Acute kidney injury in major abdominal surgery: Incidence, risk factors, pathogenesis and outcomes. Ann Intensive Care 2018;8(1):22. [CrossRef]
[Pubmed]

2.
3.
Slankamenac K, Breitenstein S, Held U, Beck-Schimmer B, Puhan MA, Clavien PA. Development and validation of a prediction score for postoperative acute renal failure following liver resection. Ann Surg 2009;520(5):720–8. [CrossRef]
[Pubmed]

4.
Lim C, Audureau E, Salloum C, et al. Acute kidney injury following hepatectomy for hepatocellular carcinoma: Incidence, risk factors and prognostic value. HPB (Oxford) 2016;18(6):540–8. [CrossRef]
[Pubmed]

5.
Tomozawa A, Ishikawa S, Shiota N, Cholvisudhi P, Makita K. Perioperative risk factors for acute kidney injury after liver resection surgery: An historical cohort study. Can J Anaesth 2015;62(7):753–61. [CrossRef]
[Pubmed]

6.
Ishikawa S, Tanaka, M, Maruyama, F, et al. Effects of acute kidney injury after liver resection on long-term outcomes. Korean J Anesthesiol 2017;70(5):527–34. [CrossRef]
[Pubmed]

7.
Cho E, Kim SC, Kim MG, Jo SK, Cho WY, Kim HK. The incidence and risk factors of acute kidney injury after hepatobiliary surgery: A prospective observational study. BMC Nephrol 2014;15:169. [CrossRef]
[Pubmed]

8.
Kambakamba P, Slankamenac K, Tschuor C, et al. Epidural analgesia and perioperative kidney function after major liver resection. Br J Surg 2015;102(7):805–12. [CrossRef]
[Pubmed]

9.
Moon YJ, Jun IG, Kim KH, Kim SO, Song JG, Hwang GS. Comparison of acute kidney injury between open and laparoscopic liver resection: Propensity score analysis. PLoS One 2017;12(10):e0186336. [CrossRef]
[Pubmed]

10.
Correa-Gallego C, Berman A, Denis SC, et al. Renal function after low central venous pressure-assisted liver resection: Assessment of 2116 cases. HPB (Oxford) 2015;17(3):258–64. [CrossRef]
[Pubmed]

11.
Hilmi IA, Damian D, Al-Khafaji A, et al. Acute kidney injury following orthotopic liver transplantation: Incidence, risk factors, and effects on patient and graft outcomes. Br J Anaesth 2015;114(6):919–26. [CrossRef]
[Pubmed]

12.
Slankamenac K, Beck-Schimmer B, Breitenstein S, Puhan MA, Clavien PA. Novel prediction score including pre- and intraoperative parameters best predicts acute kidney injury after liver surgery. World J Surg 2013;37(11):2618–28. [CrossRef]
[Pubmed]

13.
Glance LG, Wissler R, Mukamel DB, et al. Perioperative outcomes among patients with the modified metabolic syndrome who are undergoing noncardiac surgery. Anesthesiology 2010;113(4):859–72. [CrossRef]
[Pubmed]

14.
Shashaty MG, Kalkan E, Bellamy SL, et al. Computed tomography-defined abdominal adiposity is associated with acute kidney injury in critically ill trauma patients. Crit Care Med 2014;42(7):1619–28. [CrossRef]
[Pubmed]

15.
Goumenos DS, Kawar B, El Nahas M, et al. Early histological changes in the kidney of people with morbid obesity. Nephrol Dial Transplant 2009;24(12):3732–8. [CrossRef]
[Pubmed]

16.
Bell S, Dekker FW, Vadiveloo T, et al. Risk of postoperative acute kidney injury in patients undergoing orthopaedic surgery–development and validation of a risk score and effect of acute kidney injury on survival: Observational cohort study. BMJ 2015;351:h5639. [CrossRef]
[Pubmed]

17.
Kheterpal S, Tremper KK, Heung M, et al. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: Results from a national data set. Anesthesiology 2009;110(3):505–15. [CrossRef]
[Pubmed]

18.
Yokoyama Y, Schwacha MG, Samy TS, Bland KI, Chaudry IH. Gender dimorphism in immune responses following trauma and hemorrhage. Immunol Res 2002;26(1–3):63–76.
[Pubmed]

19.
Levin A, Djurdjev O, Beaulieu M, Er L. Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis 2008;52(4):661–77. [CrossRef]
[Pubmed]

20.
Pounds LL, Teodorescu VJ. Chronic kidney disease and dialysis access in women. J Vasc Surg 2013;57(4 Suppl):49S–53S.e1. [CrossRef]
[Pubmed]

21.
Shoup M, Gonen M, D'Angelica M, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg 2003;7(3):325–30.
[Pubmed]

22.
Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 2007;204(5):854–62. [CrossRef]
[Pubmed]

23.
Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 2005;16(1):162–8. [CrossRef]
[Pubmed]

24.
Bloom RD, Reese PP. Chronic kidney disease after nonrenal solid-organ transplantation. J Am Soc Nephrol 2007;18(12):3031–41. [CrossRef]
[Pubmed]

25.
Sawhney S, Mitchell M, Marks A, Fluck N, Black C. Long-term prognosis after acute kidney injury (AKI): What is the role of baseline kidney function and recovery? A systematic review. BMJ Open 2015;5(1):e006497. [CrossRef]
[Pubmed]

26.
James MT, Hemmelgarn BR, Wiebe N, et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: A cohort study. Lancet 2010;376(9758):2096–103. [CrossRef]
[Pubmed]

27.
Koyner JL. Assessment and diagnosis of renal dysfunction in the ICU. Chest 2012;141(6):1584–94. [CrossRef]
[Pubmed]

28.
van den Broek MA, Olde Damink SW, Dejong CH, et al. Liver failure after partial hepatic resection: Definition, pathophysiology, risk factors and treatment. Liver Int 2008;28(6):767–80. [CrossRef]
[Pubmed]

29.
Nadalin S, Testa G, Malagó M, et al. Volumetric and functional recovery of the liver after right hepatectomy for living donation. Liver Transpl 2004;10(8):1024–9. [CrossRef]
[Pubmed]

30.
Bredt LC, Peres LAB. Risk factors for acute kidney injury after partial hepatectomy. World J Hepatol 2017;9(18):815–22. [CrossRef]
[Pubmed]

31.
Kawamura H, Okada K, Isizu H, et al. Laparoscopic gastrectomy for early gastric cancer targeting as a less invasive procedure. Surg Endosc 2008;22(1):81–5. [CrossRef]
[Pubmed]

32.
Okholm C, Goetze JP, Svendsen LB, Achiam MP. Inflammatory response in laparoscopic vs. open surgery for gastric cancer. Scand J Gastroenterol 2014;49(9):1027–34. [CrossRef]
[Pubmed]

33.
Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol 2015;26(8):1765–76. [CrossRef]
[Pubmed]

34.
Zafrani L, Ince C. Microcirculation in acute and chronic kidney diseases. Am J Kidney Dis 2015;66(6):1083–94. [CrossRef]
[Pubmed]

35.
Sakowska M, Docherty E, Linscott D, Connor S. A change in practice from epidural to intrathecal morphine analgesia for hepato-pancreato-biliary surgery. World J Surg 2009;33(9):1802–8. [CrossRef]
[Pubmed]

36.
Lehman LW, Saeed M, Moody G, Mark R. Hypotension as a risk factor for acute kidney injury in ICU patients. Comput Cardiol (2010) 2010;37:1095–8.
[Pubmed]

37.
Tzimas P, Prout J, Papadopoulos G, Mallett SV. Epidural anaesthesia and analgesia for liver resection. Anaesthesia 2013;68(6):628–35. [CrossRef]
[Pubmed]

38.
Revie EJ, Massie LJ, McNally SJ, McKeown DW, Garden OJ, Wigmore SJ. Effectiveness of epidural analgesia following open liver resection. HPB (Oxford) 2011;13;(3):206–11. [CrossRef]
[Pubmed]

39.
Wong-Lun-Hing EM, van Dam RM, Welsh FK, et al. Postoperative pain control using continuous i.m. bupivacaine infusion plus patient-controlled analgesia compared with epidural analgesia after major hepatectomy. HPB (Oxford) 2014;16(7):601–9. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Zoka Milan - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Amy Rene Gomes - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patients for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2019 Zoka Milan et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.