| Table of Contents |  |
|
Rapid Communication
| ||||||
| Preoperative plasma cholinesterase activity in infants with congenital biliary disorder: A prospective comparative study | ||||||
| Ljiljana Popović1, Tatjana Goranović1, Josipa Kern3, Sandra Alavuk Kundović4, Marina Peklić Iveković5 | ||||||
|
1MD, PhD, Professor, Consultant Anaesthetist and Intensivist, Department of Anesthesiology, Reanimatology and Intensive Care Medicine, Children's Hospital Zagreb, Zagreb, Croatia; Professor, Faculty of Medicine, University of Zagreb, Croatia.
2MD, PhD, Senior Teaching Assistant, Consultant Anaesthetist and Intensivist, Department of Anesthesiology, Reanimatology and Intensive Care Medicine, University Department for Tumours, Sestre milosrdnice University Hospital Centre, Zagreb, Croatia; Senior Teaching Assistant, Faculty of Medicine, University of Osijek, Croatia. 3MSc, PhD, Professor, Professor of Medical Informatics, Chair of Department of Medical Statistics, Epidemiology and Medical Informatics, University of Zagreb, School of Medicine, Andrija Štampar School of Public Health, Zagreb, Croatia. 4MD, Consultant Anaesthetist and Intensivist, Department of Anesthesiology, Reanimatology and Intensive Care Medicine, Children's' Hospital Zagreb, Zagreb, Croatia. 5MD, Consultant Internist and Intensivist, Department of Internal Medicine, Zagreb University Hospital Centre, Zagreb, Croatia. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| Popovic Lj, Goranović T, Kern J, Alavuk Kundović S, Peklić Iveković M. Preoperative plasma cholinesterase activity in infants with congenital biliary disorder: A prospective comparative study. Edorium J Anesth 2016;2:20–24. |
|
Abstract
|
|
Aims:
Plasma cholinesterase (E 3.1.1.8), an enzyme synthesized in the liver, has importance to anesthetic practice for various properties. The aim of our study was to assess the preoperative liver function in terms of plasma cholinesterase activity in infants with severe liver disease.
Methods: Plasma cholinesterase activity, dibucaine number and albumin concentration were prospectively measured in 25 infants with biliary atresia before liver surgery. Twenty healthy infants undergoing elective inguinal hernia repair were included in the control group. Plasma cholinesterase activity was measured by the spectrophotometric method by Ellman. Dibucaine number was determined using benzoylcholine at 25°C in phosphate buffer in the presence of dibucaine. Serum albumin concentration was determined by immunonephelometry as described by Naathelson. Results: All infants with biliary atresia had significant lower activity of preoperative plasma cholinesterase and albumin concentration compared to the control group (p < 0.0001). In all infants dibucaine number was normal. Conclusion: This study showed that preoperative plasma cholinesterase activity in infants with biliary atresia reflects the decreased synthetic liver function as well as albumin concentration. The results indicate the possible usefulness of determining plasma cholinesterase activity preoperatively in infants with congenital biliary disorder to avoid altered response with drugs that are metabolized by plasma cholinesterase. | |
|
Keywords:
Albumin, Congenital biliary disorder, Dibucaine number, Infants, Plasma cholinesterase
| |
|
Introduction
| ||||||
|
It is well known that plasma cholinesterase (PChE, E 3.1.1.8) activity is modified not only by many physiological conditions such as age, but also by many pathological conditions, such as liver disease due to decreased its synthesis in liver [1] [2]. In addition, there is growing evidence about its property as a negative acute phase reactant in acute surgical and critical illness, independently of the modifications related to other already known factors [3]. PChE contributed important information in predicting unfavorable postoperative outcome in adult major surgery [4] [5] [6] [7][8]. PChE is also mentioned as biomarker to identify subjects at greater risk of developing postoperative delirium in elderly patients [9]. However, there is very little evidence about its property in perioperative period in children and infants [10] [11][12], and particularly in children and infants with severe liver disease [13]. The purpose of this study was to assess the preoperative liver function in terms of plasma cholinesterase activity in infants with severe liver disease. | ||||||
|
Patients and Methods
| ||||||
|
The present prospective study was approved by Hospital Ethics Committee of the Zagreb University Hospital Centre. Signed parental written informed consent was obtained from all patients. The study group included 25 infants with biliary atresia (type I:5, type II:1, type III:19) and accompanying fibro-obliterative cholangiopathy infants undergoing liver surgery. Histological examination of the liver, as well as clinical features, ultrasonography and hepatobiliary scintigraphy were performed to evaluate infants with suspected biliary atresia. The infants were aged 2–6 months at the time of surgical treatment (mean age 2.5±2.3 months). The body weight was 1980–3900 g. As a control group, 20 healthy infants, ASA (American Society of Anaesthesiologists) classified I, undergoing elective inguinal hernia repair were included. They were aged 2-6 months (mean age 2.6±2.2 months). The body weight was 3500–5300 g. In all children PChE activity, the percentage inhibition by dibucaine (dibucaine number, DN) and albumin concentration were measured prior the induction in anesthesia for scheduled surgery. For determination of PChE activity and DN vein blood samples (2.0 mL) were collected from the cannula inserted for the anesthetic procedure according to the standard anesthetic protocol. Plasma samples were stored at -20°C until analyzed. PChE activity was determined by the spectrophotometric method by Ellman [14] using butyrylcholine as substrate (Sigma Chem Co. St Louis, USA). Dibucaine number was determined at 25°C in phosphate buffer by determining the percentage inhibition of benzoylcholine hydrolysis in the presence of dibucaine (Sigma Chem Co. St. Louis, USA) [15]. Serum albumin was determined by immunonephelometry as described by Naathelson, using the Behring Nephelometer Analyzer II, Dade Behring [16]. Statistical analysis was performed with SAS statistical package (SAS Institute INC., Cary, NC, USA). Distribution of numerical data was determined with the Kolmogorov-Smirnov test and the Shapiro Wilk test of normality. Normally distributed data were expressed as mean ± standard deviation (SD), whereas not normally distributed data were presented as median with interquartile range (IQR). Categorical data were expressed as frequencies and percentages. The t test was used for comparison of PChE, DN and albumin concentration between the study and the control group. P < 0.05 was considered statistically significant. | ||||||
|
Results | ||||||
|
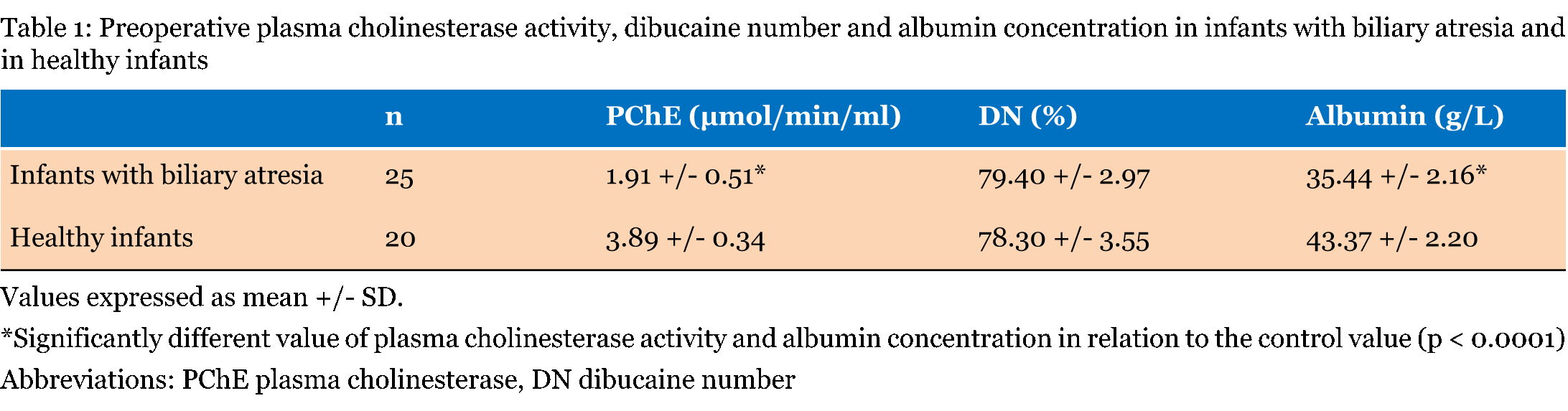
All infants with biliary atresia had significant lower activity of PChE as well as albumin concentration in relation to their control group values (Table 1). In all infants, there was no significant difference in percentage inhibition by dibucaine. | ||||||
| ||||||
|
Discussion
| ||||||
|
The results of this study showed that preoperative PChE activity in infants with biliary atresia was significantly lower compared to healthy infants. This finding was expecting and correspond well with our previous finding of lower preoperative PChE activity in children with severe liver disease when we concluded that PChE activity may reflects more sensitively the degree of synthetic liver function than albumin concentration [13]. This finding leads us to conclusion that PChE activity in similar manner as albumin concentration may be used for preoperative evaluation of liver synthesis activity in infants with liver disease. In regard to albumin concentration, it appears that the PChE activity seems to be more sensitive marker in the assessment of synthetic liver function [13]. We can possibly attribute this to the significantly shorter half-life of PChE and independent synthesis compared to albumin. The potential prognostic value of the low value of preoperative PChE was further explored in adult surgery in some studies. The low value of preoperative PChE is thought to be the predictor of non-survivors after open heart surgery in patients with severe post-hepatitis and congestive liver dysfunction [6]. Cardiac adult patients who experienced major morbidities after cardiac surgery had markedly lower levels of serum cholinesterase [7]. The univariate analysis indicated there were significant differences in preoperative PChE between the patients undergoing valvular repair surgery with and without liver dysfunction [8]. In addition, in the same group of patients multivariate logistic regression analysis also identified preoperative PChE as a predictor of mortality [8]. PChE contributed important information in predicting postoperative outcome after hepatic resection for hepatocellular cancer [4]. A value of PChE ≤ 5,900 UI/L had a sensitivity of 73% and a specificity of 67% in predicting liver-related postoperative complications (p=0.001). The multivariate analysis revealed that a value of PChE ≤ 5,900 UI/L independently predicted the risk of morbidity [4]. The recent intergroup diversity study showed the significant decrease in PChE activity among Child-Pugh A/B/C stages, in control versus Child-Pugh stage A and chronic hepatitis versus Child Pugh stage A groups [17]. PChE and Child Pugh/MELD scores showed statistically significant correlation [17]. A preoperative PChE level is an independent prognostic factor for muscle-invasive bladder cancer after radical cystectomy [5]. The five-year overall survival rates were 90.1% and 51.3% in the PChE ≥ 168 and<168 U/l groups, respectively (p<0.001). The five-year disease- free survival rates were 83.5% and 55.4% in the PChE ≥ 168 and ≤ 167 U/l groups, respectively (p<0.001) [5]. Multivariate analysis revealed that PChE was the factor most significantly associated with overall survival, and also it was significantly associated with disease-free survival [5]. We did not explored the potential prognostic value of preoperative PChE in infants in this study. However, previously we showed in 25 infants with biliary atresia that the increase of PChE activity together with the decrease of bilirubin, alkaline phosphatase and gamma-glutamyl transpeptidase showed early improvement of liver function seven days after Kasai operation [10]. We did not measured bilirubin, alkaline phosphatase and gamma-glutamyl transpeptidase in healthy control group in this study because we did not measured them routinely in healthy children and missed to include it in the study protocol during ethical approval. The lack of these data limited us in comparing the study groups in other liver function data except of PChE and albumin, as reported. In all infants we determined DN to exclude several variant genes which could result in reduced enzyme activity. Varies genotypes can produce varies clinical effects of drugs which are metabolized by PChE e.g. heterozygotes will not be sensitive to suxamethonium (depolarizing muscle relaxant) unless other contributing factors (e.g. concurrent illness are present, but homozygotes have a prevalence of ~ 1: 3000 and remain paralyzed for 2–3 hours. The fluoride resistant gene, the silent gene and other variants produce a spectrum of reduced activity of the enzyme causing variety prolongation of the action of suxamethonium; from mild prolongation to several hours of paralysis [18] [19]. In our study, in all patients DN was normal. We would like to remind that method such as dibucaine inhibition may be still useful in identifying the phenotype in plasma cholinesterase deficiency in our everyday practice since genetic testing is not widespread available. Reduced PChE activity can also occur for acquired reasons and situations like renal disease, burns, malignancy and malnutrition, all of which can be found perioperatively. Administration of drugs which share the same metabolic pathway (suxamethonium) and presence of anticholinesterase (e.g., neostigmine, edrophonium) can develop complications [19] [20] . Mivacurium is non-depolarizing muscle relaxant which is also metabolized by PChE. Individuals deficient in the enzyme may develop prolonged muscle paralysis [20]. It is recommended to use neuromonitoring in patients with PChE deficiency [21] [22] because of higher risk of being awakened while paralyzed and being distressed during emergence from anesthesia. Plasma cholinesterase also partially metabolizes remifentanil, a selective opioid agonist. Low enzyme activity can complicate their use [23]. The small number of patients may be an additional limitation of this study. However, this number correlates with the fact that biliary atresia is rare condition with an incidence of 1:15,000 births each year [24]. Despite this limitation, we believe that the findings of this study may be helpful in clinical settings during preoperative evaluation and planning anesthetic management of infants with congenital biliary disorder to avoid altered response with drugs that are metabolized by plasma cholinesterase. | ||||||
|
Conclusion
| ||||||
|
This study showed that preoperative plasma cholinesterase (PChE) activity reflects the decreased synthetic liver function in infants with biliary atresia as well as albumin concentration. Plasma cholinesterase activity could be a useful preoperative index of liver function in infants with congenital biliary disorder. Further studies can determine its potential prognostic value in perioperative period in this specific group of patients. | ||||||
|
Acknowledgements
| ||||||
|
This study was supported by the grant of Ministry of Science, Education and Sports of the Republic of Croatia. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Ljljana Popović – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Tatjana Goranović – Conception and design, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Josipa Kern – Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Sandra Alavuk Kundović – Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Marina Peklić Iveković – Acquisition of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2016 Ljljana Popović et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|