| Table of Contents |  |
|
Case Series
| |||||
| Rare reasons of central venous catheter failure: A case series | |||||
| Theodoros Aslanidis1, Athanasios Kontos1, Vasilios Ourailoglou1 | |||||
|
1Intensive Care Unit, Department of Anesthesiology and Intensive Care Medicine, "AHEPA" General University Hospital, Thessaloniki, Greece.
| |||||
| |||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article: |
| Aslanidis T, Kontos A, Ourailoglou V. Rare reasons of central venous catheter failure: A case series. Edorium J Intensive Care 2014;1:1–3. |
|
Abstract
|
|
Introduction:
Central venous catheter is one of the most essential and frequent equipment used in intensive care unit. Several patients who receive these catheters may present mechanical, thrombotic or infectious complications.
Case Series: We hereby report two very rare reasons of central venous catheter failure in two critically ill patients. Conclusion: The reported cases point out the importance of the thorough knowledge and control of every medical device we use. | |
|
Keywords:
Central venous catheter, Complication, Intensive care, Catheter failure
| |
|
Introduction
| ||||||
|
Central venous catheters (CVC) allow measurement of variables that cannot be measured accurately by non-invasive means and allow delivery of medications and nutritional support that cannot be given safely through peripheral venous catheters. Unfortunately, the use of central venous catheters may be associated with adverse events that are both hazardous to patients and expensive to treat. We present the management of two cases in our intensive care unit who presented rare complication of CVC failure. | ||||||
|
Case Series
| ||||||
|
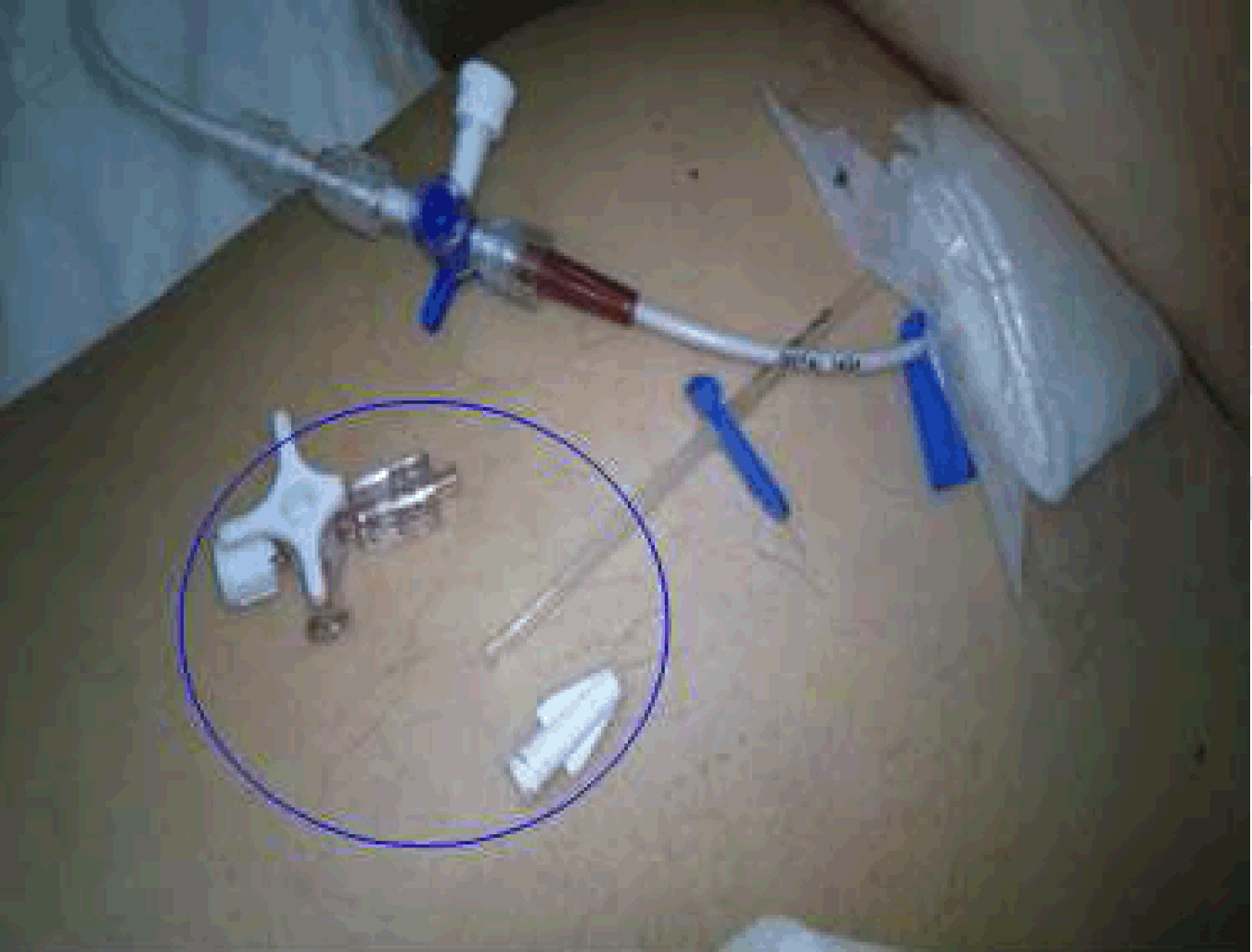
Case 1: A 74-year-old male patient (body weight 88 kg, height 178 cm, BMI 28.08 kg/cm,2) was admitted to our intensive care unit (ICU) after spontaneous intracerebral hemorrhage. The patient had a 3-lumen CVC in the right internal jugular vein which was inserted in the emergency department. On the third day of his stay in the ICU an elective removal of the CVC was conducted and a new 2-lumen antimicrobial-impregnated CVC was inserted in the left femoral vein without any problem. The patient's therapeutic regimen included several medications, sedation agents, fluids but no vasopressors administered through the CVC. In the afternoon of the fifth day of its insertion moisture was spotted close to the catheter insertion site. All lines connected to the catheter were thoroughly checked for leaks, but the problem was not determined. The CVC insertion site was also dry. Nevertheless, new intravenous systems and new dressing were applied. An hour later a larger leak was presented from the second lumen. Administration of drugs and fluids through this lumen was interrupted and the CVC was rechecked. A very unusual technical reason for the problem was soon detected (Figure 1). A new CVC was inserted uneventfully and the patient's status was not affected by the incident. | ||||||
| ||||||
|
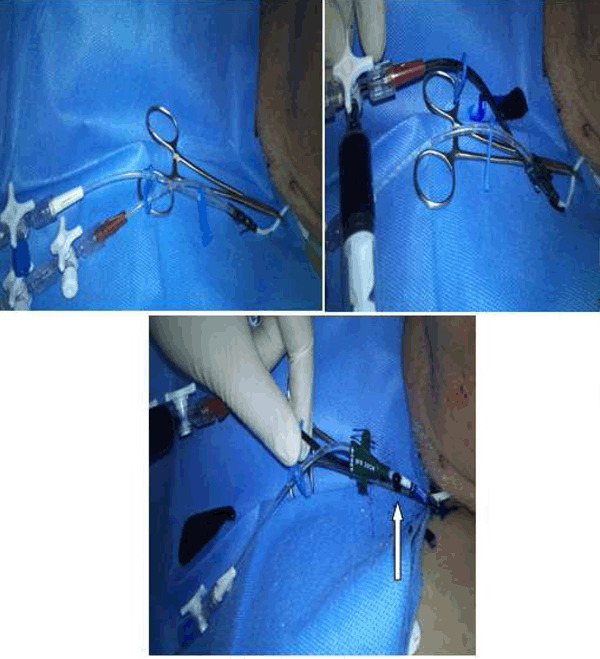
Case 2: A 76-year-old male patient was admitted to ICU for status epilepticus. The patient had a 2-lumen CVC in the right internal jugular vein which was inserted in the emergency department. After four days of uneventful functioning of the CVC, fluid leakage was spotted around the insertion point. All infusion were stopped temporarily and all lines were checked using methylene blue (Figure 2A-C). A rupture is spotted at the side of the proximal tube. A new CVC was inserted uneventfully without affecting the overall patient's status. | ||||||
| ||||||
|
Discussion
| ||||||
|
In the United States only , physicians insert more than 5 million central venous catheters every year resulting in 15 million central vascular catheter (CVC) days (i.e, the total number of days of exposure to CVCs among all patients in the selected population during the selected time period) [1] [2]. However, the latter are often associated not only with increased morbidity, mortality but also with increased cost and duration of hospitalization. Mechanical complications are reported to occur in 5–19% of patients, infectious complications in 5–26%, and thrombotic complications in 2–26% [2]. These complications are related to the insertion site and technique, the experience of the physician, the characteristics of the patient, the use of the catheter (i.e. hemodialysis, chemotherapy) and the existence of an emergency situation [2] [3] [4] . Complication referring to the CVC material during their use is an extremely rare event due to the very strict standards in the procedure of their manufacturing and the clearly formed guidelines for their maintenance [1] [2] [5] [6] [7] [8] . To our knowledge this is the first reported case of CVC failure, after four days of use without problems and despite the full compliance of the nursing staff with the guidelines for the use and maintenance of this particular device: manufacturer guidelines and rules for device stabilization, infection and safety compliance, dressing and insertion site care, maintaining patency procedures and infusion equipment care. Our patients did not suffer any further complications, due to properly and on time change of the malfunctioning catheter. | ||||||
|
Conclusion
| ||||||
|
The reported case points out the importance of the thorough knowledge and control of every medical device we use and alerts us towards such rare events that could possibly happen in more crucial situations, i.e., during resuscitation, surgery, administering drugs essential for the patient's stability, etc. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Theodoros Aslanidis – Substantial contributions to conception and design, Acquisition of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Athanasios Kontos – Substantial contributions to conception and design, Acquisition of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Vasilios Ourailoglou – Substantial contributions to conception and design, Acquisition of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. Cases presented as abstract in 12th Conference of Society of anesthesiology and intensive care of Northern Greece. |
|
Copyright
© 2014 Theodoros Aslanidis et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|